Theoretical Investigation on the "ON-OFF" Mechanism of a Fluorescent Probe for Thiophenols: Photoinduced Electron Transfer and Intramolecular Charge Transfer
- PMID: 37836764
- PMCID: PMC10574459
- DOI: 10.3390/molecules28196921
Theoretical Investigation on the "ON-OFF" Mechanism of a Fluorescent Probe for Thiophenols: Photoinduced Electron Transfer and Intramolecular Charge Transfer
Abstract
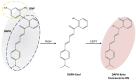
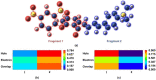
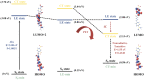
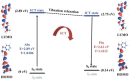
In this study, the sensing mechanism of (2E,4E)-5-(4-(dimethylamino)phenyl)-1-(2-(2,4dinitrophenoxy)phenyl)penta-2,4-dien-1-one (DAPH-DNP) towards thiophenols was investigated by density functional theory (DFT) and time-dependent DFT (TD-DFT). The DNP group plays an important role in charge transfer excitation. Due to the typical donor-excited photo-induced electron transfer (d-PET) process, DAPH-DNP has fluorescence quenching behavior. After the thiolysis reaction between DAPH-DNP and thiophenol, the hydroxyl group is released, and DAPH is generated with the reaction showing strong fluorescence. The fluorescence enhancement of DAPH is not caused by an excited-state intramolecular proton transfer (ESIPT) process. The potential energy curves (PECs) show that DAPH-keto is less stable than DAPH-enol. The frontier molecular orbitals (FMOs) of DAPH show that the excitation process is accompanied by intramolecular charger transfer (ICT), and the corresponding character of DAPH was further confirmed by hole-electron and interfragment charge transfer (IFCT) analysis methods. Above all, the sensing mechanism of the turn-on type probe DAPH-DNP towards thiophenol is based on the PET mechanism.
Keywords: ESIPT; d-PET; frontier molecular orbital; thiophenol.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; collection, analyses, or interpretation of data; writing of the manuscript, or decision to publish the results.
Figures

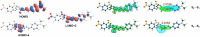





References
-
- Ji H.-F., Shen L., Zhang H.-Y. Theoretical Reinvestigation of Opposite Electronic Effects on Bond Lengths in Thiophenols and Thiophenolic Radicals. J. Struct. Chem. 2005;46:347–351. doi: 10.1007/s10947-006-0052-y. - DOI
-
- dos Santos D.J.V.A., Newton A.S., Bernardino R., Guedes R.C. Substituent Effects on O–H and S–H Bond Dissociation Enthalpies of Disubstituted Phenols and Thiophenols. Int. J. Quantum Chem. 2008;108:754–761. doi: 10.1002/qua.21522. - DOI
-
- Xiao M.-M., Ren H., Liu T.-Z., Li Z.-Y., Wang J.-Z., Miao J.-Y., Zhao B.-X. Two Fluorescent Turn-on Probes for Detecting Thiophenols in Environmental Water and in Living Cell Imaging. Microchem. J. 2022;175:107220. doi: 10.1016/j.microc.2022.107220. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

