Functionalized Calixarenes as Promising Antibacterial Drugs to Face Antimicrobial Resistance
- PMID: 37836797
- PMCID: PMC10574364
- DOI: 10.3390/molecules28196954
Functionalized Calixarenes as Promising Antibacterial Drugs to Face Antimicrobial Resistance
Abstract
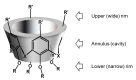
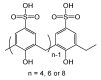
Since the discovery of polyphenolic resins 150 years ago, the study of polymeric compounds named calix[n]arene has continued to progress, and those skilled in the art perfectly know now how to modulate this phenolic ring. Consequently, calix[n]arenes are now used in a large range of applications and notably in therapeutic fields. In particular, the calix[4]arene exhibits multiple possibilities for regioselective polyfunctionalization on both of its rims and offers researchers the possibility of precisely tuning the geometry of their structures. Thus, in the crucial research of new antibacterial active ingredients, the design of calixarenes finds its place perfectly. This review provides an overview of the work carried out in this aim towards the development of intrinsically active prodrogues or metallic calixarene complexes. Out of all the work of the community, there are some excellent activities emerging that could potentially place these original structures in a very good position for the development of new active ingredients.
Keywords: antibacterial activity; antibacterial drugs; antimicrobial resistance; bacterial infections; calixarenes; functionalized calixarenes; nosocomial infections; small molecules.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





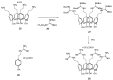

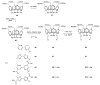

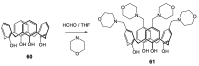

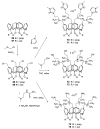











References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

