Phase-separation multiphase flow: preliminary application to analytical chemistry
- PMID: 37837525
- PMCID: PMC10766728
- DOI: 10.1007/s44211-023-00442-1
Phase-separation multiphase flow: preliminary application to analytical chemistry
Abstract
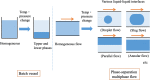
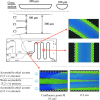
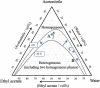
A two-phase separation mixed solution can undergo phase separation from one phase to two phases (i.e., upper and lower phases) in a batch vessel in response to changes in temperature and/or pressure. This phase separation is reversible. When the mixed solution undergoes a phase change while being fed into a microspace region, a dynamic liquid-liquid interface is formed, leading to a multiphase structure. This flow is called a phase-separation multiphase flow. Annular flow in a microspace, which is one such phase-separation multiphase flow, is interesting and has been applied to chromatography, extraction, reaction fields, and mixing. Here, research papers related to phase-separation multiphase flows-ranging from the discovery of the phenomenon to basic and technical research from the viewpoint of analytical science-are reviewed. In addition, the development of a new separation mode in a high-performance liquid chromatography system based on phase-separation multiphase flow is introduced.
Keywords: High-performance liquid chromatography (HPLC); Phase-separation mode; Phase-separation multiphase flow; Tube radial distribution chromatography (TRDC); Tube radial distribution extraction (TRDE); Tube radial distribution flow (TRDF); Tube radial distribution mixing (TRDM); Tube radial distribution reaction (TRDR).
© 2023. The Author(s).
Figures

















References
-
- Tsukagoshi K. Kagaku. 2016;71:74.
-
- Tsukagoshi K. Bunrigijutsu. 2019;49:24.
-
- Reuss FF. Proc. Imp. Soc. Nat. Moscow. 1809;2:327.
-
- Hagen G. Ann. Phys. Chem. 1839;46:423. doi: 10.1002/andp.18391220304. - DOI
-
- Poiseuille JLR. C R. 1841;11:961.
Publication types
LinkOut - more resources
Full Text Sources

