Residual circulating tumor DNA after adjuvant chemotherapy effectively predicts recurrence of stage II-III gastric cancer
- PMID: 37837629
- PMCID: PMC10693304
- DOI: 10.1002/cac2.12494
Residual circulating tumor DNA after adjuvant chemotherapy effectively predicts recurrence of stage II-III gastric cancer
Abstract
Background: Circulating tumor DNA (ctDNA) is a promising biomarker for predicting relapse in multiple solid cancers. However, the predictive value of ctDNA for disease recurrence remains indefinite in locoregional gastric cancer (GC). Here, we aimed to evaluate the predictive value of ctDNA in this context.
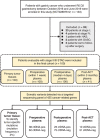
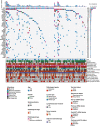
Methods: From 2016 to 2019, 100 patients with stage II/III resectable GC were recruited in this prospective cohort study (NCT02887612). Primary tumors were collected during surgical resection, and plasma samples were collected perioperatively and within 3 months after adjuvant chemotherapy (ACT). Somatic variants were captured via a targeted sequencing panel of 425 cancer-related genes. The plasma was defined as ctDNA-positive only if one or more variants detected in the plasma were presented in at least 2% of the primary tumors.
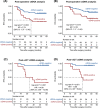
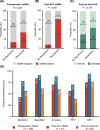
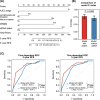
Results: Compared with ctDNA-negative patients, patients with positive postoperative ctDNA had moderately higher risk of recurrence [hazard ratio (HR) = 2.74, 95% confidence interval (CI) = 1.37-5.48; P = 0.003], while patients with positive post-ACT ctDNA showed remarkably higher risk (HR = 14.99, 95% CI = 3.08-72.96; P < 0.001). Multivariate analyses indicated that both postoperative and post-ACT ctDNA positivity were independent predictors of recurrence-free survival (RFS). Moreover, post-ACT ctDNA achieved better predictive performance (sensitivity, 77.8%; specificity, 90.6%) than both postoperative ctDNA and serial cancer antigen. A comprehensive model incorporating ctDNA for recurrence risk prediction showed a higher C-index (0.78; 95% CI = 0.71-0.84) than the model without ctDNA (0.71; 95% CI = 0.64-0.79; P = 0.009).
Conclusions: Residual ctDNA after ACT effectively predicts high recurrence risk in stage II/III GC, and the combination of tissue-based and circulating tumor features could achieve better risk prediction.
Keywords: chemotherapy; ctDNA; gastric cancer; postoperative; recurrence.
© 2023 The Authors. Cancer Communications published by John Wiley & Sons Australia, Ltd on behalf of SUN YAT-SEN UNIVERSITY CANCER CENTER.
Conflict of interest statement
The authors disclose no conflicts.
Figures
References
-
- Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA: a cancer journal for clinicians. 2021;71(3):209–249. - PubMed
-
- Ajani JA, D'Amico TA, Bentrem DJ, Chao J, Cooke D, Corvera C, et al. Gastric Cancer, Version 2.2022, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2022;20(2):167–192. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
- 2019B020227002/Science and Technology Program of Guangdong
- 2019-I2M-5-036/CAMS Innovation Fund for Medical Sciences (CIFMS)
- 82061160373/International Cooperation and Exchanges National Natural Science Foundation of China
- 81872011/National Natural Science Foundation of China
- 2018014/Sun Yat-sen University Clinical Research 5010 Program
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

