Biophysical characterization of chloride intracellular channel 6 (CLIC6)
- PMID: 37838179
- PMCID: PMC10641671
- DOI: 10.1016/j.jbc.2023.105349
Biophysical characterization of chloride intracellular channel 6 (CLIC6)
Abstract
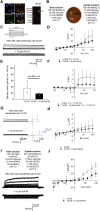
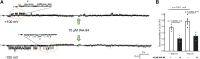
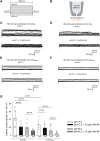
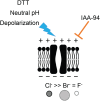
Chloride intracellular channels (CLICs) are a family of proteins that exist in soluble and transmembrane forms. The newest discovered member of the family CLIC6 is implicated in breast, ovarian, lung gastric, and pancreatic cancers and is also known to interact with dopamine-(D(2)-like) receptors. The soluble structure of the channel has been resolved, but the exact physiological role of CLIC6, biophysical characterization, and the membrane structure remain unknown. Here, we aimed to characterize the biophysical properties of this channel using a patch-clamp approach. To determine the biophysical properties of CLIC6, we expressed CLIC6 in HEK-293 cells. On ectopic expression, CLIC6 localizes to the plasma membrane of HEK-293 cells. We established the biophysical properties of CLIC6 by using electrophysiological approaches. Using various anions and potassium (K+) solutions, we determined that CLIC6 is more permeable to chloride-(Cl-) as compared to bromide-(Br-), fluoride-(F-), and K+ ions. In the whole-cell configuration, the CLIC6 currents were inhibited after the addition of 10 μM of IAA-94 (CLIC-specific blocker). CLIC6 was also found to be regulated by pH and redox potential. We demonstrate that the histidine residue at 648 (H648) in the C terminus and cysteine residue in the N terminus (C487) are directly involved in the pH-induced conformational change and redox regulation of CLIC6, respectively. Using qRT-PCR, we identified that CLIC6 is most abundant in the lung and brain, and we recorded the CLIC6 current in mouse lung epithelial cells. Overall, we have determined the biophysical properties of CLIC6 and established it as a Cl- channel.
Keywords: IAA-94; anion transport; chloride channel; pH-regulation; redox-regulation.
Copyright © 2023 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Conflict of interest The authors declare that they have no conflicts of interest with the contents of this article.
Figures



References
-
- Jentsch T.J., Stein V., Weinreich F., Zdebik A.A. Molecular structure and physiological function of chloride channels. Physiol. Rev. 2002;82:503–568. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

