Piloting an ICU follow-up clinic to improve health-related quality of life in ICU survivors after a prolonged intensive care stay (PINA): feasibility of a pragmatic randomised controlled trial
- PMID: 37838669
- PMCID: PMC10576359
- DOI: 10.1186/s12871-023-02255-1
Piloting an ICU follow-up clinic to improve health-related quality of life in ICU survivors after a prolonged intensive care stay (PINA): feasibility of a pragmatic randomised controlled trial
Abstract
Background: ICU survivors often suffer from prolonged physical and mental impairments resulting in the so called "Post-Intensive Care Syndrome" (PICS). The aftercare of former ICU patients affected by PICS in particular has not been addressed sufficiently in Germany so far. The aim of this study was to evaluate the feasibility of a pragmatic randomised trial (RCT) comparing an intensive care unit (ICU) follow-up clinic intervention to usual care.
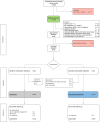
Methods: This pilot study in a German university hospital evaluated the feasibility of a pragmatic RCT. Patients were assigned in a 1:1 ratio to an ICU follow-up clinic intervention or to usual care. The concept of this follow-up clinic was previously developed in a participatory process with patients, next of kin, health care professionals and researchers. We performed a process evaluation and determined acceptability, fidelity, completeness of measurement instruments and practicality as feasibility outcomes. The RCT's primary outcome (health-related quality of life) was assessed six months after ICU discharge by means of the physical component scale of the Short-Form-12 self-report questionnaire.
Results: The pilot study was conducted from June 2020 to May 2021 with 21 and 20 participants in the intervention and control group. Principal findings related to feasibility were 85% consent rate (N = 48), 62% fidelity rate, 34% attrition rate (N = 41) and 77% completeness of outcome measurements. The primary effectiveness outcome (health-related quality of life) could be measured in 93% of participants who completed the study (N = 27). The majority of participants (85%) needed assistance with follow-up questionnaires (practicality). Median length of ICU stay was 13 days and 85% (N = 41) received mechanical ventilation, median Sequential Organ Failure Assessment Score was nine. Six-month follow-up assessment was planned for all study participants and performed for 66% (N = 41) of the participants after 197 days (median).
Conclusion: The participatory developed intervention of an ICU follow-up clinic and the pragmatic pilot RCT both seem to be feasible. We recommend to start a pragmatic RCT on the effectiveness of the ICU follow-up clinic.
Trial registration: ClinicalTrials.gov US NLM, NCT04186468, Submission: 02/12/2019, Registration: 04/12/2019, https://clinicaltrials.gov/ct2/show/NCT04186468.
Keywords: Complex intervention; Critical care; ICU follow-up clinic; Mechanical ventilation; Pilot study; Post-intensive care syndrome (PICS).
© 2023. BioMed Central Ltd., part of Springer Nature.
Conflict of interest statement
The authors declare no competing interests.
Figures

Similar articles
-
Piloting an ICU follow-up clinic to improve health-related quality of life in ICU survivors after a prolonged intensive care stay (PINA): study protocol for a pilot randomised controlled trial.Pilot Feasibility Stud. 2021 Mar 30;7(1):90. doi: 10.1186/s40814-021-00796-1. Pilot Feasibility Stud. 2021. PMID: 33785064 Free PMC article.
-
IMPACT-ICU feasibility study: pragmatic mixed-methods randomised controlled trial of a follow-up care intervention for survivors of critical illness and caregivers.BMJ Open. 2025 Jan 2;15(1):e086799. doi: 10.1136/bmjopen-2024-086799. BMJ Open. 2025. PMID: 39753245 Free PMC article.
-
A protocol for a pilot randomised controlled trial of an Early Psychiatric Assessment, Referral, and Intervention Study (EPARIS) for intensive care patients.PLoS One. 2023 Jun 29;18(6):e0287470. doi: 10.1371/journal.pone.0287470. eCollection 2023. PLoS One. 2023. PMID: 37384627 Free PMC article.
-
Prevalence of Post-intensive care syndrome among intensive care unit-survivors and its association with intensive care unit length of stay: Systematic review and meta-analysis.PLoS One. 2025 May 8;20(5):e0323311. doi: 10.1371/journal.pone.0323311. eCollection 2025. PLoS One. 2025. PMID: 40338918 Free PMC article.
-
Screening tools for post-intensive care syndrome and post-traumatic symptoms in intensive care unit survivors: A scoping review.Aust Crit Care. 2023 Sep;36(5):863-871. doi: 10.1016/j.aucc.2022.09.007. Epub 2022 Dec 1. Aust Crit Care. 2023. PMID: 36464526
Cited by
-
ICU follow-up services and their impact on post-intensive care syndrome: a scoping review protocol.BMJ Open. 2024 Nov 12;14(11):e089824. doi: 10.1136/bmjopen-2024-089824. BMJ Open. 2024. PMID: 39532379 Free PMC article.
-
Post intensive care syndrome: A review of clinical symptoms, evaluation, intervention.Heliyon. 2024 May 17;10(10):e31278. doi: 10.1016/j.heliyon.2024.e31278. eCollection 2024 May 30. Heliyon. 2024. PMID: 38803859 Free PMC article. Review.
-
The implementation and evaluation of a family-led novel intervention for delirium prevention and management in adult critically ill patients: A mixed-methods pilot study.Nurs Crit Care. 2025 Jul;30(4):e13210. doi: 10.1111/nicc.13210. Epub 2024 Dec 1. Nurs Crit Care. 2025. PMID: 39617957 Free PMC article.
References
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Medical

