Recruiting women with ductal carcinoma in situ to a randomised controlled trial: lessons from the LORIS study
- PMID: 37838682
- PMCID: PMC10576350
- DOI: 10.1186/s13063-023-07703-4
Recruiting women with ductal carcinoma in situ to a randomised controlled trial: lessons from the LORIS study
Abstract
Background: The LOw RISk DCIS (LORIS) study was set up to compare conventional surgical treatment with active monitoring in women with ductal carcinoma in situ (DCIS). Recruitment to trials with a surveillance arm is known to be challenging, so strategies to maximise patient recruitment, aimed at both patients and recruiting centres, were implemented.
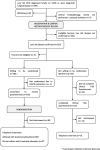
Methods: Women aged ≥ 46 years with a histologically confirmed diagnosis of non-high-grade DCIS were eligible for 1:1 randomisation to either surgery or active monitoring. Prior to randomisation, all eligible women were invited to complete: (1) the Clinical Trials Questionnaire (CTQ) examining reasons for or against participation, and (2) interviews exploring in depth opinions about the study information sheets and film. Women agreeing to randomisation completed validated questionnaires assessing health status, physical and mental health, and anxiety levels. Hospital site staff were invited to communication workshops and refresher site initiation visits to support recruitment. Their perspectives on LORIS recruitment were collected via surveys and interviews.
Results: Eighty percent (181/227) of eligible women agreed to be randomised. Over 40% of participants had high anxiety levels at baseline. On the CTQ, the most frequent most important reasons for accepting randomisation were altruism and belief that the trial offered the best treatment, whilst worries about randomisation and the influences of others were the most frequent most important reasons for declining. Most women found the study information provided clear and useful. Communication workshops for site staff improved knowledge and confidence but only about half said they themselves would join LORIS if eligible. The most common recruitment barriers identified by staff were low numbers of eligible patients and patient preference.
Conclusions: Recruitment to LORIS was challenging despite strategies aimed at both patients and site staff. Ensuring that recruiting staff support the study could improve recruitment in similar future trials.
Trial registration: ISRCTN27544579, prospectively registered on 22 May 2014.
Keywords: DCIS; LORIS; Patient interviews; Patient preference; Randomisation; Trials.
© 2023. BioMed Central Ltd., part of Springer Nature.
Conflict of interest statement
Not applicable.
Figures
References
-
- NHS Breast Screening Programme, Association of Breast Surgery. An audit of screen detected breast cancers for the screening year April 2020 to March 2021. 2022.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources