Serum immune checkpoint profiling identifies soluble CD40 as a biomarker for pancreatic cancer
- PMID: 37838778
- PMCID: PMC10576756
- DOI: 10.1038/s41698-023-00459-9
Serum immune checkpoint profiling identifies soluble CD40 as a biomarker for pancreatic cancer
Abstract
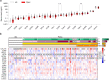
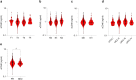
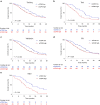
Pancreatic ductal adenocarcinoma (PDAC) responds poorly to systemic treatment, including new immunotherapeutic approaches. Biomarkers are urgently needed for early disease detection, patient stratification for treatment, and response prediction. The role of soluble CD40 (sCD40) is unknown in PDAC. In this study, we performed a quantitative multiplex analysis of 17 immune checkpoint proteins in serum samples from patients with various stages of PDAC in a discovery study (n = 107) and analyzed sCD40 by ELISA in a validation study (n = 317). Youden's J statistic was used for diagnostic cut-off optimization. A Cox proportional hazards regression model was applied in an empiric approach for prognostic threshold optimization. Kaplan-Meier estimator and multivariable Cox regression analyses were used for survival analysis. sCD40 was significantly increased in the serum of patients with PDAC compared to healthy cohorts and patients with IPMN. In the validation cohort, the area under the receiver operating characteristic (ROC) c-statistic was 0.8, and combining sCD40 with CA19-9 yielded a c-statistic of 0.95. sCD40 levels were independent of the tumor stage. However, patients who received neoadjuvant chemotherapy had significantly lower sCD40 levels than those who underwent upfront surgery. Patients with a sCD40 level above the empirical threshold of 0.83 ng/ml had a significantly reduced overall survival with a hazard ratio of 1.4. This observation was pronounced in patients after neoadjuvant chemotherapy. Collectively, soluble CD40 may be considered as both a diagnostic and prognostic non-invasive biomarker in PDAC.
© 2023. Nature Publishing Group UK.
Conflict of interest statement
The authors declare no competing interests.
Figures

References
-
- American Cancer Society. Cancer Facts & Figures 2022, Vol. 80 (American Cancer Society, 2022).
-
- Rahib L, et al. Projecting cancer incidence and deaths to 2030: the unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer Res. 2014;74:2913–2921. - PubMed
-
- Sharma P, et al. The next decade of immune checkpoint therapy. Cancer Discov. 2021;11:838–857. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

