Adipose stromal cells bioproducts as cell-free therapies: manufacturing and therapeutic dose determine in vitro functionality
- PMID: 37840135
- PMCID: PMC10577984
- DOI: 10.1186/s12967-023-04602-9
Adipose stromal cells bioproducts as cell-free therapies: manufacturing and therapeutic dose determine in vitro functionality
Abstract
Background: Extracellular vesicles (EV) are considered a cell-free alternative to mesenchymal stromal cell (MSC) therapy. Numerous reports describe the efficacy of EV in conferring immunomodulation and promoting angiogenesis, yet others report these activities to be conveyed in EV-free bioproducts. We hypothesized that this discrepancy may depend either on the method of isolation or rather the relative impact of the individual bioactive components within the MSC secretome.
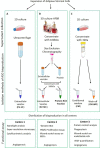
Methods: To answer this question, we performed an inter-laboratory study evaluating EV generated from adipose stromal cells (ASC) by either sequential ultracentrifugation (UC) or size-exclusion chromatography (SEC). The effect of both EV preparations on immunomodulation and angiogenesis in vitro was compared to that of the whole secretome and of the EV-free protein fraction after SEC isolation.
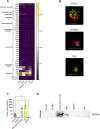
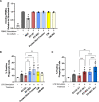
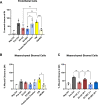
Results: In the current study, neither the EV preparations, the secretome or the protein fraction were efficacious in inhibiting mitogen-driven T cell proliferation. However, EV generated by SEC stimulated macrophage phagocytic activity to a similar extent as the secretome. In turn, tube formation and wound healing were strongly promoted by the ASC secretome and protein fraction, but not by EV. Within the secretome/protein fraction, VEGF was identified as a potential driver of angiogenesis, and was absent in both EV preparations.
Conclusions: Our data indicate that the effects of ASC on immunomodulation and angiogenesis are EV-independent. Specific ASC-EV effects need to be dissected for their use as cell-free therapeutics.
Keywords: Angiogenesis; Immune modulation; Mesenchymal stromal cells; Secretome.
© 2023. BioMed Central Ltd., part of Springer Nature.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
References
-
- Colombo M, Raposo G, Théry C. Biogenesis, secretion, and intercellular interactions of exosomes and other extracellular vesicles. Annu Rev Cell Dev Biol. 2014;30:255–289. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

