Diagnostic delay of multiple sclerosis: prevalence, determinants and consequences
- PMID: 37840276
- PMCID: PMC10580682
- DOI: 10.1177/13524585231197076
Diagnostic delay of multiple sclerosis: prevalence, determinants and consequences
Abstract
Background: Early diagnosis and treatment of patients with multiple sclerosis (MS) are associated with better outcomes; however, diagnostic delays remain a major problem.
Objective: Describe the prevalence, determinants and consequences of delayed diagnoses.
Methods: This single-centre ambispective study analysed 146 adult relapsing-remitting MS patients (2016-2021) for frequency and determinants of diagnostic delays and their associations with clinical, cognitive, imaging and biochemical measures.
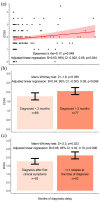
Results: Diagnostic delays were identified in 77 patients (52.7%), including 42 (28.7%) physician-dependent cases and 35 (24.0%) patient-dependent cases. Diagnosis was delayed in 22 (15.1%) patients because of misdiagnosis by a neurologist. A longer diagnostic delay was associated with trends towards greater Expanded Disability Status Scale (EDSS) scores (B = 0.03; p = 0.034) and greater z-score of the blood neurofilament light chain (B = 0.35; p = 0.031) at the time of diagnosis. Compared with patients diagnosed at their first clinical relapse, patients with a history of >1 relapse at diagnosis (n = 63; 43.2%) had a trend towards greater EDSS scores (B = 0.06; p = 0.006) and number of total (B = 0.13; p = 0.040) and periventricular (B = 0.06; p = 0.039) brain lesions.
Conclusion: Diagnostic delays in MS are common, often determined by early misdiagnosis and associated with greater disease burden.
Keywords: Delayed diagnosis; brain lesion; cerebrospinal fluid; disability; magnetic resonance imaging; misdiagnosis; multiple sclerosis; neurofilament.
Conflict of interest statement
Declaration of Conflicting InterestsThe author(s) declared the following potential conflicts of interest with respect to the research, authorship and/or publication of this article: A.A. L.N., A.M.M., J.L., V.R., A.B., K.N. and L.F. have nothing to disclose. M.V. received speaker honoraria and consultant fees from Biogen, Novartis, Roche, Sanofi/Genzyme, TEVA, as well as support for research activities from Biogen Idec. B.S. received compensation for travelling and conference fees from Novartis, Sanofi, Biogen, Roche and Merck as well as support for research activities from Biogen. B.M. and T.K. are the employees of Siemens Healthineers International AG, Switzerland. J.K. received financial support for research activities from Biogen Idec. D.S. received financial support for conference travel from Novartis, Biogen, Merck, Bayer and Janssen-Cilag. M.A. received financial support for conference travel from Novartis, Genzyme, Merck Serono, Biogen Idec and Roche. K.V. received compensation for travelling, and conference fees from Biogen, Novartis and Merck. J.M. received compensation for travelling, conference fees and speaker honoraria from Sanofi Genzyme, Biogen, Novartis and Merck. P.D. received speaker honoraria from Orphalan and consultant fees from Alexion Pharmaceuticals, Inc. E.K.H. received speaker honoraria and consultant fees from Biogen Idec, Merck Serono, Novartis, Genzyme, Teva, Actelion and Receptos, as well as support for research activities from Biogen Idec and Merck Serono. D.H. received compensation for travel, speaker honoraria and consultant fees from Biogen Idec, Novartis, Merck Serono, Bayer Shering and Teva, as well as support for research activities from Biogen Idec. J.K. received speaker fees, research support, travel support and/or served on the advisory boards by ECTRIMS, Swiss MS Society, Swiss National Research Foundation, (320030_160221), University of Basel, Bayer, Biogen, Celgene, Genzyme, Merck, Novartis, Protagen AG, Roche and Teva. T.U. received financial support for conference travel and honoraria from Biogen Idec, Novartis, Roche, Genzyme and Merck Serono, as well as support for research activities from Biogen Idec and Sanofi.
Figures



References
-
- Giovannoni G, Butzkueven H, Dhib-Jalbut S, et al. Brain health: time matters in multiple sclerosis. Mult Scler Relat Disord 2016; 9(suppl 1): S5–S48. - PubMed
-
- Clinical guideline [CG186] multiple sclerosis in adults: management. 2019. https://www.nice.org.uk/guidance/cg186
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

