A quest for the stereo-electronic requirements for selective agonism for the neurotrophin receptors TrkA and TrkB in 17-spirocyclic-dehydroepiandrosterone derivatives
- PMID: 37840771
- PMCID: PMC10568017
- DOI: 10.3389/fnmol.2023.1244133
A quest for the stereo-electronic requirements for selective agonism for the neurotrophin receptors TrkA and TrkB in 17-spirocyclic-dehydroepiandrosterone derivatives
Abstract
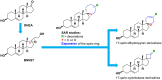
Introduction: The neurotrophin system plays a pivotal role in the development, morphology, and survival of the nervous system, and its dysregulation has been manifested in numerous neurodegenerative and neuroinflammatory diseases. Neurotrophins NGF and BDNF are major growth factors that prevent neuronal death and synaptic loss through binding with high affinity to their specific tropomyosin-related kinase receptors namely, TrkA and TrkB, respectively. The poor pharmacokinetic properties prohibit the use of neurotrophins as therapeutic agents. Our group has previously synthesized BNN27, a prototype small molecule based on dehydroepiandrosterone, mimicking NGF through the activation of the TrkA receptor.
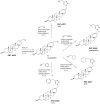
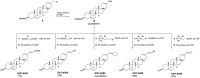
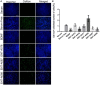
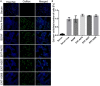
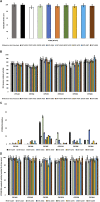
Methods: To obtain a better understanding of the stereo-electronic requirements for selective activation of TrkA and TrkB receptors, 27 new dehydroepiandrosterone derivatives bearing a C17-spiro-dihydropyran or cyclobutyl moiety were synthesized. The new compounds were evaluated for their ability (a) to selectively activate the TrkA receptor and its downstream signaling kinases Akt and Erk1/2 in PC12 cells, protecting these cells from serum deprivation-induced cell death, and (b) to induce phosphorylation of TrkB and to promote cell survival under serum deprivation conditions in NIH3T3 cells stable transfected with the TrkB receptor and primary cortical astrocytes. In addition the metabolic stability and CYP-mediated reaction was assessed.
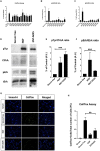
Results: Among the novel derivatives, six were able to selectively protect PC12 cells through interaction with the TrkA receptor and five more to selectively protect TrkB-expressing cells via interaction with the TrkB receptor. In particular, compound ENT-A025 strongly induces TrkA and Erk1/2 phosphorylation, comparable to NGF, and can protect PC12 cells against serum deprivation-induced cell death. Furthermore, ENT-A065, ENT-A066, ENT-A068, ENT-A069, and ENT-A070 showed promising pro-survival effects in the PC12 cell line. Concerning TrkB agonists, ENT-A009 and ENT-A055 were able to induce phosphorylation of TrkB and reduce cell death levels in NIH3T3-TrkB cells. In addition, ENT-A076, ENT-A087, and ENT-A088 possessed antiapoptotic activity in NIH-3T3-TrkB cells exclusively mediated through the TrkB receptor. The metabolic stability and CYP-mediated reaction phenotyping of the potent analogs did not reveal any major liabilities.
Discussion: We have identified small molecule selective agonists of TrkA and TrkB receptors as promising lead neurotrophin mimetics for the development of potential therapeutics against neurodegenerative conditions.
Keywords: DHEA derivatives; TrkA; TrkB; neurodegeneration; neuroprotection; neurotrophin mimetics; receptor agonists; signaling.
Copyright © 2023 Narducci, Charou, Rogdakis, Zota, Bafiti, Zervou, Katsila, Gravanis, Prousis, Charalampopoulos and Calogeropoulou.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures








References
-
- Barter Z. E., Bayliss M. K., Beaune P. H., Boobis A. R., Carlie D. J., Edwards R. J., et al. . (2007). Scaling factors for the extrapolation of in vivo metabolic drug clearance from in vitro data: reaching a consensus on values of human microsomal protein and hepatocellularity per gram of liver. Curr. Drug. Meta. 8 33–45. 10.2174/138920007779315053 - DOI - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

