First-line tepotinib for a very elderly patient with metastatic NSCLC harboring MET exon 14 skipping mutation and high PD-L1 expression
- PMID: 37840886
- PMCID: PMC10568218
- DOI: 10.33393/dti.2023.2626
First-line tepotinib for a very elderly patient with metastatic NSCLC harboring MET exon 14 skipping mutation and high PD-L1 expression
Abstract
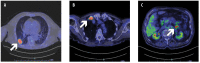
Optimal treatment for metastatic non-small cell lung cancer (NSCLC) with mesenchymal epithelial transition gene (MET) exon 14 skipping mutation has not been established yet. MET inhibitors were demonstrated to be effective and tolerated in patients with this condition, while evidence on safety and efficacy of immunotherapy and/or chemotherapy in this population is limited. Here we report the case of an 86-year-old male with metastatic NSCLC harboring MET exon 14 skipping mutation and with high programmed cell death ligand 1 (PD-L1) expression (tumor proportion score ≥50%). The patient received the MET inhibitor tepotinib as first-line treatment, achieving a partial response, with G2 peripheral edema as adverse event that was successfully managed with temporary discontinuation, dose reduction, diuretics and physical therapy. After 31 months, the patient is still receiving tepotinib, with an ongoing response. Tepotinib is a valuable therapeutic option for first-line treatment of older patients with NSCLC harboring MET exon 14 skipping mutation, even in the presence of high PD-L1 expression.
Keywords: Elderly; First-line treatment; MET 14-exon skipping mutation; MET inhibitor; Non-small cell lung cancer; Tepotinib.
Copyright © 2023, The Authors.
Conflict of interest statement
Conflict of interest: The authors declare no conflict of interest.
Figures

References
Publication types
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

