Nasal Absorption Enhancement of Mometasone Furoate Nanocrystal Dispersions
- PMID: 37841023
- PMCID: PMC10573391
- DOI: 10.2147/IJN.S430952
Nasal Absorption Enhancement of Mometasone Furoate Nanocrystal Dispersions
Abstract
Purpose: We designed a 0.05% mometasone furoate (MF) nanocrystal dispersion and investigated whether the application of MF nanocrystals in nasal formulations enhanced local absorption compared to traditional nasal MF formulations (CA-MF).
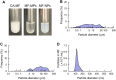
Methods: MF nanocrystal dispersions (MF-NPs) were prepared by bead milling MF microcrystal dispersions (MF-MPs) consisting of MF, 2-hydroxypropyl-β-cyclodextrin, methylcellulose, and purified water. Pluronic F-127 combined with methylcellulose, Pluronic F-68, or carbopol was used as a base for in situ gelation (thickener). MF concentrations were measured using high-performance liquid chromatography, and nasal absorption of MF was evaluated in 6 week-old male Institute of Cancer Research (ICR) mice.
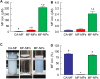
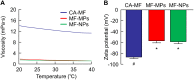
Results: The particle size range of MF prepared with the bead mill treatment was 80-200 nm, and the nanoparticles increased the local absorption of MF, which was higher than that of CA-MF and MF-MPs. In addition, unlike the results obtained in the small intestine and corneal tissue, the high absorption of nanocrystalline MF in the nasal mucosa was related to a pathway that was not derived from energy-dependent endocytosis. Moreover, the application of the in situ gelling system attenuated the local absorption of MF-NPs, owing to a decrease in drug diffusion in the dispersions.
Conclusion: We found that nanoparticulation of MF enhances local intranasal absorption, and nasal bioavailability is higher than that of CA-MF. In addition, we demonstrate that viscosity regulation is an important factor in the design of nasal formulations based on MF nanocrystals. These findings provide insights for the design of novel nanomedicines with enhanced nasal bioavailability.
Keywords: drug delivery; in situ gel; mometasone furoate; nanocrystal; nasal absorption.
© 2023 Masuda et al.
Conflict of interest statement
The authors report no conflicts of interest in this work.
Figures




Similar articles
-
Formulation and Evaluation of Thermoreversible In Situ Nasal Gels Containing Mometasone Furoate for Allergic Rhinitis.AAPS PharmSciTech. 2017 Oct;18(7):2673-2682. doi: 10.1208/s12249-017-0747-8. Epub 2017 Mar 9. AAPS PharmSciTech. 2017. PMID: 28281209
-
Development of Niosomal Vesicles Loaded Mometasone Furoate Gel for Transdermal Delivery and its Evaluation.Recent Adv Drug Deliv Formul. 2023;17(4):300-313. doi: 10.2174/0126673878259437231031114907. Recent Adv Drug Deliv Formul. 2023. PMID: 37974444
-
Pharmacokinetics of intranasal mometasone in the fixed-dose combination GSP301 versus two monotherapy intranasal mometasone formulations.Allergy Asthma Proc. 2018 May 1;39(3):232-239. doi: 10.2500/aap.2018.39.4134. Allergy Asthma Proc. 2018. PMID: 29669668 Clinical Trial.
-
Mometasone furoate nasal spray: a review of safety and systemic effects.Drug Saf. 2007;30(4):317-26. doi: 10.2165/00002018-200730040-00004. Drug Saf. 2007. PMID: 17408308 Review.
-
Mometasone furoate nasal spray for the treatment of asthma.Expert Opin Investig Drugs. 2016 Aug;25(8):999-1004. doi: 10.1080/13543784.2016.1192124. Epub 2016 Jun 1. Expert Opin Investig Drugs. 2016. PMID: 27218300 Review.
Cited by
-
Dermal formulation based on carbopol and Gum Arabic improves skin retention of indomethacin.PLoS One. 2025 Jun 10;20(6):e0326051. doi: 10.1371/journal.pone.0326051. eCollection 2025. PLoS One. 2025. PMID: 40493628 Free PMC article.
-
Nanocrystals and nanosuspensions: an exploration from classic formulations to advanced drug delivery systems.Drug Deliv Transl Res. 2024 Dec;14(12):3438-3451. doi: 10.1007/s13346-024-01559-0. Epub 2024 Mar 7. Drug Deliv Transl Res. 2024. PMID: 38451440 Free PMC article. Review.
-
Correlation among post-surgery recurrence of CRSwNP and TCM syndromes and tissue inflammatory cell infiltration type: a study protocol.Syst Rev. 2024 May 30;13(1):145. doi: 10.1186/s13643-024-02562-9. Syst Rev. 2024. PMID: 38816878 Free PMC article.
References
-
- Compalati E, Penagos M, Henley K, Canonica GW. Allergy prevalence survey by the World Allergy Organization. Allergy Clin Immunol Int J World Allergy Org. 2007;19:82–90.
-
- Asher MI, Montefort S, Bjӧrkstén B, et al. Worldwide time trends in the prevalence of symptoms of asthma, allergic rhinoconjunctivitis, and eczema in childhood: ISAAC Phases One and Three repeat multicountry cross-sectional surveys. Lancet. 2006;368(9537):733–743. doi:10.1016/S0140-6736(06)69283-0 - DOI - PubMed
-
- van Cauwenberge P, Bachert C, Passalacqua G, et al. Consensus statement on the treatment of allergic rhinitis. European Academy of Allergology and Clinical Immunology. Allergy. 2000;55(2):116–134. - PubMed
-
- Bousquet J, van Cauwenberge P, Khaltaev N, et al. Allergic rhinitis and its impact on asthma. J Allergy Clin Immunol. 2001;108(5 Suppl):S147–S334. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

