Quercetin and 5-Fu Loaded Chitosan Nanoparticles Trigger Cell-Cycle Arrest and Induce Apoptosis in HCT116 Cells via Modulation of the p53/p21 Axis
- PMID: 37841142
- PMCID: PMC10569019
- DOI: 10.1021/acsomega.3c03933
Quercetin and 5-Fu Loaded Chitosan Nanoparticles Trigger Cell-Cycle Arrest and Induce Apoptosis in HCT116 Cells via Modulation of the p53/p21 Axis
Abstract
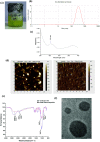
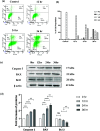
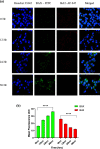
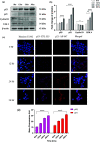
Nanoparticles (NPs) are encapsulating agents that exist in the nanometer range. They can be classified into different classes based on their properties, shapes, or sizes. Metal NPs, fullerenes, polymeric NPs, ceramic NPs, and luminescent nanoporous hybrid materials are only a few examples. This study explored the anticancer potential of quercetin and 5-fluorouracil-encapsulated chitosan nanoparticles (CS-5-FU-QCT NPs). The nanoparticles were prepared by ionic gelation, and their efficacy and mechanism of action were examined. CS-5-FU-QCT NPs were characterized using dynamic light scattering (DLS), atomic force microscopy (AFM), UV-visible spectroscopy, and Fourier transform infrared spectroscopy (FTIR); cytotoxicity was analyzed using an MTT assay. Cells were treated with CS-5-FU-QCT NPs and incubated for 12, 24, and 36 h, and apoptosis analysis (using Annexin V/FITC), cell-cycle analysis, Western blotting, and confocal microscopic analysis were performed. Biophysical analysis revealed that the CS-5-FU-QCT NPs fall in the range of 300-400 nm with a near-spherical shape. The in vitro drug release profile indicates sustained release of drugs over a period of about 36 h. The cytotoxicity of CS-5-FU-QCT NPs was more prominent in HCT116 cells than in other cancer cells. This particular nanoformulation caused G0/G1 phase cell-cycle arrest in HCT116 cells and induced intracellular ROS generation, thereby causing apoptosis. It also downregulated Bcl2, cyclin D1, and Cdk4 and upregulated BAX, p53, and p21, causing cell-cycle arrest and apoptosis. In summary, CS-5-FU-QCT NPs hindered proliferation of HCT116 cells via ROS generation and altered the expression of key proteins in the p53/p21 axis and apoptotic machinery in a time-dependent manner.
© 2023 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures


Similar articles
-
In vitro and in vivo anticancer efficacy potential of Quercetin loaded polymeric nanoparticles.Biomed Pharmacother. 2018 Oct;106:1513-1526. doi: 10.1016/j.biopha.2018.07.106. Epub 2018 Jul 25. Biomed Pharmacother. 2018. PMID: 30119227
-
Physical PEGylation Enhances The Cytotoxicity Of 5-Fluorouracil-Loaded PLGA And PCL Nanoparticles.Int J Nanomedicine. 2019 Nov 28;14:9259-9273. doi: 10.2147/IJN.S223368. eCollection 2019. Int J Nanomedicine. 2019. PMID: 31819428 Free PMC article.
-
Zinc-Phosphate Nanoparticles as a Novel Anticancer Agent: An In Vitro Evaluation of Their Ability to Induce Apoptosis.Biol Trace Elem Res. 2020 Nov;198(1):109-117. doi: 10.1007/s12011-020-02054-6. Biol Trace Elem Res. 2020. PMID: 32006202
-
Targeted Anticancer Drug Delivery Using Chitosan, Carbon Quantum Dots, and Aptamers to Deliver Ganoderic Acid and 5-Fluorouracil.Chem Biodivers. 2023 Sep;20(9):e202300659. doi: 10.1002/cbdv.202300659. Epub 2023 Sep 5. Chem Biodivers. 2023. PMID: 37548485
-
The anti-cancer effect of chitosan/resveratrol polymeric nanocomplex against triple-negative breast cancer; an in vitro assessment.IET Nanobiotechnol. 2023 Apr;17(2):91-102. doi: 10.1049/nbt2.12108. Epub 2022 Nov 24. IET Nanobiotechnol. 2023. PMID: 36420812 Free PMC article.
Cited by
-
Nanoparticle-delivered quercetin exhibits enhanced efficacy in eliminating iron-overloaded senescent chondrocytes.Nanomedicine (Lond). 2024;19(26):2159-2170. doi: 10.1080/17435889.2024.2393074. Epub 2024 Sep 4. Nanomedicine (Lond). 2024. PMID: 39229808
-
Galangin-loaded chitosan nanoparticles inhibit the cell cycle progression and cell proliferation by modulating cyclin-dependent kinases in breast cancer cells.Naunyn Schmiedebergs Arch Pharmacol. 2025 Jun 17. doi: 10.1007/s00210-025-04358-7. Online ahead of print. Naunyn Schmiedebergs Arch Pharmacol. 2025. PMID: 40526278
-
Quercetin in Oncology: A Phytochemical with Immense Therapeutic Potential.Curr Drug Targets. 2024;25(11):740-751. doi: 10.2174/0113894501292466240627050638. Curr Drug Targets. 2024. PMID: 38988154 Review.
-
A Systematic Review: Quercetin-Secondary Metabolite of the Flavonol Class, with Multiple Health Benefits and Low Bioavailability.Int J Mol Sci. 2024 Nov 11;25(22):12091. doi: 10.3390/ijms252212091. Int J Mol Sci. 2024. PMID: 39596162 Free PMC article.
-
Quercetin: A Potential Polydynamic Drug.Molecules. 2023 Dec 17;28(24):8141. doi: 10.3390/molecules28248141. Molecules. 2023. PMID: 38138630 Free PMC article. Review.
References
-
- Khan I.; Saeed K.; Khan I. Nanoparticles: Properties, applications and toxicities. Arabian J. Chem. 2019, 12 (7), 908–931. 10.1016/j.arabjc.2017.05.011. - DOI
-
- Patra J. K.; Das G.; Fraceto L. F.; et al. Nano based drug delivery systems: Recent developments and future prospects 10 Technology 1007 Nanotechnology 03 Chemical Sciences 0306 Physical Chemistry (incl. Structural) 03 Chemical Sciences 0303 Macromolecular and Materials Chemistry 11 Medical and Health Sciences 1115 Pharmacology and Pharmaceutical Sciences 09 Engineering 0903 Biomedical Engineering Prof Ueli Aebi, Prof Peter Gehr. J. Nanobiotechnology. 2018, 16 (1), 7110.1186/s12951-018-0392-8. - DOI - PMC - PubMed
-
- WHO . Cancer. https://www.who.int/news-room/fact-sheets/detail/cancer.
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

