Binding of Stimuli-Responsive Ruthenium Aqua Complexes with 9-Ethylguanine
- PMID: 37841177
- PMCID: PMC10569010
- DOI: 10.1021/acsomega.3c05343
Binding of Stimuli-Responsive Ruthenium Aqua Complexes with 9-Ethylguanine
Abstract
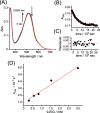
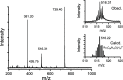
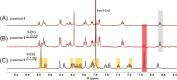
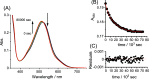
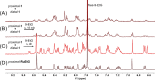
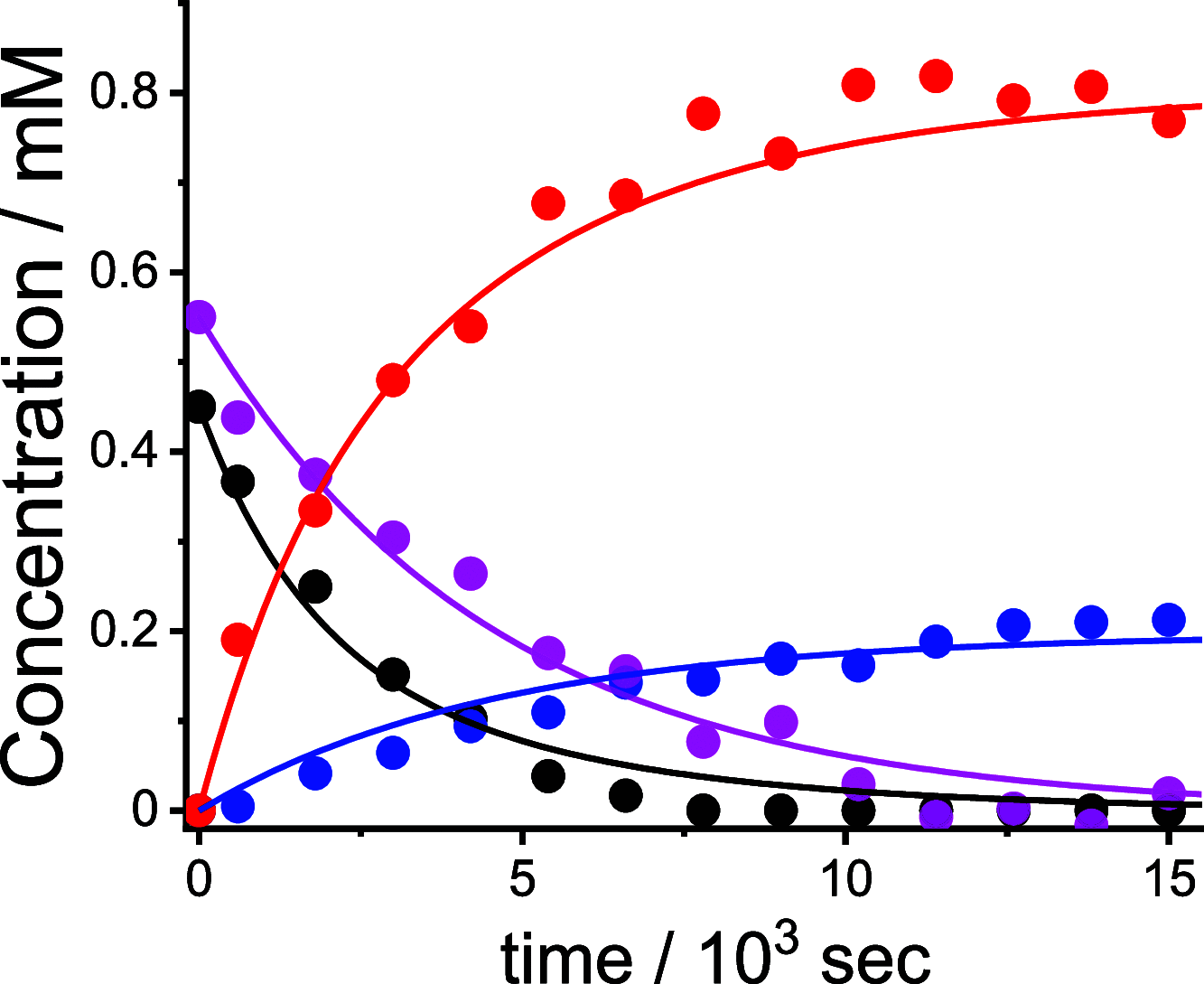
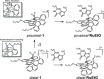
Stimuli-responsive ruthenium complexes proximal- and distal-[Ru(C10tpy)(C10pyqu) OH2]2+ (proximal-1 and distal-1; C10tpy = 4'-decyloxy-2,2':6',2″-terpyridine and C10pyqu = 2-[2'-(6'-decyloxy)-pyridyl]quinoline) were experimentally studied for adduct formation with a model DNA base. At 303 K, proximal-1 exhibited 1:1 adduct formation with 9-ethylguanine (9-EtG) to yield proximal-[Ru(C10tpy)(C10pyqu)(9-EtG)]2+ (proximal-RuEtG). Rotation of the guanine ligand on the ruthenium center was sterically hindered by the presence of an adjacent quinoline moiety at 303 K. Results from 1H NMR measurements indicated that photoirradiation of a proximal-RuEtG solution caused photoisomerization to distal-RuEtG, whereas heating of proximal-RuEtG caused ligand substitution to proximal-1. The distal isomer of the aqua complex, distal-1, was observed to slowly revert to proximal-1 at 303 K. In the presence of 9-EtG, distal-1 underwent thermal back-isomerization to proximal-1 and adduct formation to distal-RuEtG. Kinetic analysis of 1H NMR measurements showed that adduct formation between proximal-1 and 9-EtG was 8-fold faster than that between distal-1 and 9-EtG. This difference may be attributed to intramolecular hydrogen bonding and steric repulsion between the aqua ligand and the pendant moiety of the bidentate ligand..
© 2023 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures




References
-
- Eisenreich F.; Kathan M.; Dallmann A.; Ihrig S. P.; Schwaar T.; Schmidt B. M.; Hecht S. A photoswitchable catalyst system for remote-controlled (co) polymerization in situ. Nat. Catal. 2018, 1 (7), 516–522. 10.1038/s41929-018-0091-8. - DOI
LinkOut - more resources
Full Text Sources

