Comparison of the profiles of first-line PD-1/PD-L1 inhibitors for advanced NSCLC lacking driver gene mutations: a systematic review and Bayesian network meta-analysis
- PMID: 37841212
- PMCID: PMC10568994
- DOI: 10.1177/20406223231189224
Comparison of the profiles of first-line PD-1/PD-L1 inhibitors for advanced NSCLC lacking driver gene mutations: a systematic review and Bayesian network meta-analysis
Abstract
Background: Numerous first-line immune checkpoint inhibitors (ICI) were developed for patients with advanced non-small cell lung cancer (NSCLC) lacking driver gene mutations. However, this group consists of a heterogeneous patient population, for whom the optimal therapeutic choice is yet to be confirmed.
Objective: To identify the best first-line immunotherapy regimen for overall advanced NSCLC patients and different subgroups.
Design: Systematic review and Bayesian network meta-analysis (NMA).
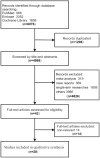
Methods: We searched several databases to retrieve relevant literature. We performed Bayesian NMA for the overall survival (OS), progression-free survival (PFS), objective response rate (ORR), and treatment-related adverse events (tr-AEs) with a grade equal or more than 3 (grade ⩾ 3 tr-AEs). Subgroup analysis was conducted according to programed death ligand 1 (PD-L1) levels, histologic type, central nervous system (CNS) metastases and tobacco use history.
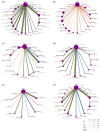
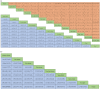
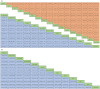
Results: For the PD-L1 non-selective patients, sintilimab plus chemotherapy (sinti-chemo) provided the best OS [hazard ratio (HR) = 0.59, 95% confidence interval (CI):0.42-0.83]. Nivolumab plus bevacizumab plus chemotherapy (nivo-bev-chemo) was comparable to atezolizumab plus bevacizumab plus chemotherapy (atezo-bev-chemo) in prolonging PFS (HR = 0.99, 95% CI: 0.51-1.91). Atezo-bev-chemo remarkably elevated the ORR than chemotherapy (OR = 3.13, 95% CI: 1.51-6.59). Subgroup analysis showed pembrolizumab plus chemotherapy (pembro-chemo) ranked first in OS in subgroups of PD-L1 < 1%, non-squamous, no CNS metastases, with or without smoking history, and ranked second in OS in subgroups of PD-L1 ⩾ 1% and PD-L1 1-49%. Cemiplimab and sugemalimab plus chemotherapy ranked first in OS and PFS for squamous subgroup, respectively. For patients with CNS metastases, nivolumab plus ipilimumab plus chemotherapy (nivo-ipili-chemo) and camrelizumab plus chemotherapy provided the best OS and PFS, respectively.
Conclusions: Sinti-chemo and nivo-bev-chemo were two effective first-line regimens ranked first in OS and PFS for overall patients, respectively. Pembro-chemo was favorable for patients in subgroups of PD-L1 < 1%, PD-L1 ⩾ 1%, PD-L1 1-49%, non-squamous, no CNS metastases, with or without smoking history. Addition of bevacizumab consistently provided with favorable PFS results in patients of all PD-L1 levels. Cemiplimab was the best option in squamous subgroup and nivo-ipili-chemo in CNS metastases subgroup due to their advantages in OS.
Keywords: PD-1/PD-L1 inhibitors; bevacizumab; immunotherapy; network meta-analysis; non-small cell lung cancer.
Plain language summary
First-line PD-1/PD-L1 inhibitors for advanced NSCLC patients lacking driver gene mutations Patients with advance non-small cell lung cancer (NSCLC) lacking driver gene mutations are a group of heterogeneous people. Although numerous therapeutic regimens were developed, the optimal choice for advanced NSCLC patients and specific subgroups is yet to be identified.We conducted a Bayesian network meta-analysis with the currently available data, and performed subgroup analyses according to programed death ligand 1 (PD-L1) levels, histologic type, CNS metastases and tobacco use history.Our key findings were as follows: (1) in non-selective PD-L1 groups, sinti-chemo and pembro-chemo provided the best OS outcome; nivo-bev-chemo and atezo-bev-chemo resulted in the most prolonged PFS; atezo-bev-chemo and pembro-chemo yielded significantly improved ORR; (2) pembro-chemo was favorable for patients in subgroups of PD-L1 < 1%, PD-L1 ⩾ 1%, PD-L1 1–49%, non-squamous, no CNS metastases, with or without smoking history; (3) immunochemotherapies involving anti-PD-1 agents generally exhibited potential advantages over those with anti-PD-L1 drugs; (4) addition of anti-VEGF drugs to immunochemotherapies consistently provided with favorable PFS results in advanced NSCLC patients with or without PD-L1 selection; (5) in patients with squamous NSCLC, cemiplimab and suge-chemo were the optimal drugs for improving OS and PFS, respectively; in patients with non-squamous NSCLC, pembro-chemo provided the best OS, while nivo-bev-chemo, atezo-bev-chemo, sinti-chemo, and pembro-chemo showed comparable advantages in improving PFS; (6) for patients with CNS metastases, nivo-ipili-chemo and camre-chemo provided the best OS and PFS, respectively.Our findings provide evidence for a more precise selection of first-line immunotherapy regimen for advanced NSCLC patients.
© The Author(s), 2023.
Conflict of interest statement
The authors declare that there is no conflict of interest.
Figures


Similar articles
-
Comparison of efficacy and safety of PD-1/PD-L1 combination therapy in first-line treatment of advanced NSCLC: an updated systematic review and network meta-analysis.Clin Transl Oncol. 2024 Oct;26(10):2488-2502. doi: 10.1007/s12094-024-03442-3. Epub 2024 Apr 16. Clin Transl Oncol. 2024. PMID: 38625495
-
Efficacy and safety of immune checkpoint inhibitors for advanced non-small cell lung cancer with or without PD-L1 selection: A systematic review and network meta-analysis.Chin Med J (Engl). 2023 Sep 20;136(18):2156-2165. doi: 10.1097/CM9.0000000000002750. Epub 2023 Aug 18. Chin Med J (Engl). 2023. PMID: 37596898 Free PMC article.
-
Cost-effectiveness of first-line immunotherapies for advanced non-small cell lung cancer.Cancer Med. 2023 Apr;12(7):8838-8850. doi: 10.1002/cam4.5632. Epub 2023 Jan 18. Cancer Med. 2023. PMID: 36653947 Free PMC article.
-
Single or combined immune checkpoint inhibitors compared to first-line platinum-based chemotherapy with or without bevacizumab for people with advanced non-small cell lung cancer.Cochrane Database Syst Rev. 2020 Dec 14;12(12):CD013257. doi: 10.1002/14651858.CD013257.pub2. Cochrane Database Syst Rev. 2020. Update in: Cochrane Database Syst Rev. 2021 Apr 30;4:CD013257. doi: 10.1002/14651858.CD013257.pub3. PMID: 33316104 Free PMC article. Updated.
-
Single or combined immune checkpoint inhibitors compared to first-line platinum-based chemotherapy with or without bevacizumab for people with advanced non-small cell lung cancer.Cochrane Database Syst Rev. 2021 Apr 30;4(4):CD013257. doi: 10.1002/14651858.CD013257.pub3. Cochrane Database Syst Rev. 2021. PMID: 33930176 Free PMC article.
Cited by
-
PD-(L)1 inhibitors plus bevacizumab and chemotherapy as first-line therapy in PD-L1-negative metastatic lung adenocarcinoma: a real-world data.J Cancer Res Clin Oncol. 2024 Mar 18;150(3):135. doi: 10.1007/s00432-024-05637-1. J Cancer Res Clin Oncol. 2024. PMID: 38499838 Free PMC article.
-
Association Between Cutaneous Immune-Related Adverse Events and Efficacy of Immune Checkpoint Inhibitors in Advanced Non-Small Cell Lung Cancer.J Clin Med. 2025 Apr 6;14(7):2499. doi: 10.3390/jcm14072499. J Clin Med. 2025. PMID: 40217946 Free PMC article.
References
-
- Sung H, Ferlay J, Siegel RL, et al.. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2021; 71: 209–249. - PubMed
-
- Duma N, Santana-Davila R, Molina JR. Non-small cell lung cancer: epidemiology, screening, diagnosis, and treatment. Mayo Clin Proc 2019; 94: 1623–1640. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

