Combined regulation of pro-inflammatory cytokines production by STAT3 and STAT5 in a model of B. pertussis infection of alveolar macrophages
- PMID: 37841236
- PMCID: PMC10569487
- DOI: 10.3389/fimmu.2023.1254276
Combined regulation of pro-inflammatory cytokines production by STAT3 and STAT5 in a model of B. pertussis infection of alveolar macrophages
Abstract
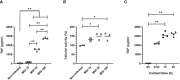
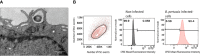
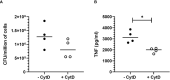
Bordetella pertussis is a highly contagious respiratory pathogen responsible for whooping-cough or pertussis. Despite high vaccination coverage worldwide, this gram-negative bacterium continues to spread among the population. B. pertussis is transmitted by aerosol droplets from an infected individual to a new host and will colonize its upper respiratory tract. Alveolar macrophages (AMs) are effector cells of the innate immune system that phagocytose B. pertussis and secrete both pro-inflammatory and antimicrobial mediators in the lungs. However, understanding their role in B. pertussis pathogenesis at the molecular level is hampered by the limited number of primary AMs that can be collected in vivo. In order to decipher the regulation of innate response induced by B. pertussis infection, we used for the first time self-renewing, non-transformed cells, called Max Planck Institute (MPI) cells, which are phenotypically and functionally very close to pulmonary AMs. Using optimized infection conditions, we characterized the entry and the clearance of B. pertussis within MPI macrophages. We showed that under these conditions, MPI cells exhibit a pro-inflammatory phenotype with the production of TNF, IL-1β, IL-6 and MIP-2α, similarly to primary AMs purified from broncho-alveolar fluids of mice. In addition, we explored the yet uncharacterized role of the signal transduction activator of transcription (STAT) proteins family in the innate immune response to B. pertussis infection and showed for the first time the parallel regulation of pro-inflammatory cytokines by STAT3 and STAT5 in MPI macrophages infected by B. pertussis. Altogether, this work highlights the interest of using MPI cells for experiments optimization and preliminary data acquisition to understand B. pertussis interaction with AMs, and thus significantly reduce the number of animals to be sacrificed.
Keywords: Bordetella pertussis; STAT proteins; alveolar macrophages; cytokines; infection.
Copyright © 2023 Khiter, Kherrouche, Dubois, Slupek, Petit, Debrie, Cauchi, Barois, Rouanet and Mielcarek.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


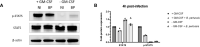
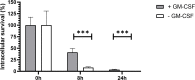

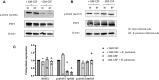
References
-
- Abbafati C, Abbas KM, Abbasi-Kangevari M, Abd-Allah F, Abdelalim A, Abdollahi M, et al. Global burden of 369 diseases and injuries in 204 countries and territories, 1990–2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet (2020) 396(10258):1204–22. doi: 10.1016/S0140-6736(20)30925-9 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

