The clinical relevance of OSM in inflammatory diseases: a comprehensive review
- PMID: 37841259
- PMCID: PMC10570509
- DOI: 10.3389/fimmu.2023.1239732
The clinical relevance of OSM in inflammatory diseases: a comprehensive review
Abstract
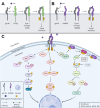
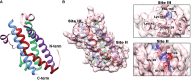
Oncostatin M (OSM) is a pleiotropic cytokine involved in a variety of inflammatory responses such as wound healing, liver regeneration, and bone remodeling. As a member of the interleukin-6 (IL-6) family of cytokines, OSM binds the shared receptor gp130, recruits either OSMRβ or LIFRβ, and activates a variety of signaling pathways including the JAK/STAT, MAPK, JNK, and PI3K/AKT pathways. Since its discovery in 1986, OSM has been identified as a significant contributor to a multitude of inflammatory diseases, including arthritis, inflammatory bowel disease, lung and skin disease, cardiovascular disease, and most recently, COVID-19. Additionally, OSM has also been extensively studied in the context of several cancer types including breast, cervical, ovarian, testicular, colon and gastrointestinal, brain,lung, skin, as well as other cancers. While OSM has been recognized as a significant contributor for each of these diseases, and studies have shown OSM inhibition is effective at treating or reducing symptoms, very few therapeutics have succeeded into clinical trials, and none have yet been approved by the FDA for treatment. In this review, we outline the role OSM plays in a variety of inflammatory diseases, including cancer, and outline the previous and current strategies for developing an inhibitor for OSM signaling.
Keywords: cancer; cytokine; drug development; inflammatory diseases; metastasis; oncostatin M (OSM); oncostatin M receptor beta (OSMRβ); therapeutics.
Copyright © 2023 Wolf, Pruett, Lighter and Jorcyk.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

