Carbonyl Allylation and Crotylation: Historical Perspective, Relevance to Polyketide Synthesis, and Evolution of Enantioselective Ruthenium-Catalyzed Hydrogen Auto-Transfer Processes
- PMID: 37841289
- PMCID: PMC10569400
- DOI: 10.1055/s-0042-1751420
Carbonyl Allylation and Crotylation: Historical Perspective, Relevance to Polyketide Synthesis, and Evolution of Enantioselective Ruthenium-Catalyzed Hydrogen Auto-Transfer Processes
Abstract
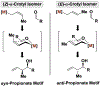
The evolution of methods for carbonyl allylation and crotylation of alcohol proelectrophiles culminating in the design of iodide-bound ruthenium-JOSIPHOS catalysts is prefaced by a brief historical perspective on asymmetric carbonyl allylation and its relevance to polyketide construction. Using gaseous allene or butadiene as precursors to allyl- or crotylruthenium nucleophiles, respectively, new capabilities for carbonyl allylation and crotylation have been unlocked, including stereo- and site-selective methods for the allylation and crotylation of 1,3-diols and related polyols.
Keywords: Allene; Allylation; Butadiene; Feedstock; Polyketide; Ruthenium.
Conflict of interest statement
Conflict of Interest There are no conflicts to declare.
Figures




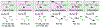
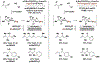
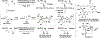
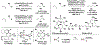
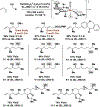

References
-
- Saytzeff M; Saytzeff A; Sorokin B Chem. Ber 1876, 9, 33.
- Kanonnikoff J; Saytzeff A Ann. Chem. Pharm 1877, 185, 148.
- Saytzeff M; Saytzeff A Chem. Pharm 1877, 185, 151.
- Béhal A; Sommelet M Academie des Sciences 1904, 138, 92.
- Mikhailov BM; Bubnov YN Izv. Akad. Nauk SSSR, Ser. Khim 1964, 1874.
- König K; Neumann WP Tetrahedron Lett. 1967, 8, 493
- Hosomi A, Sakurai H, Tetrahedron Lett. 1976, 16, 1295.
- Okude Y; Hirano S; Hiyama T; Nozaki HJ Am. Chem. Soc 1977, 99, 3179.
-
- For selected examples of chiral reagents for asymmetric carbonyl allylmetalation, see:
- Herold T; Hoffmann RW Angew. Chem. Int. Ed 1978, 17, 768.
- Hoffmann RW; Herold T Chem. Ber 1981, 114, 375.
- Brown HC; Jadhav PK J. Am. Chem. Soc 1983, 105, 2092.
- Brown HC; Bhat KS J. Am. Chem. Soc 1986, 108, 293. - PubMed
- Brown HC; Bhat KS J. Am. Chem. Soc 1986, 108, 5919. - PubMed
- Roush WR; Walts AE; Hoong LK J. Am. Chem. Soc 1985, 107, 8186.
- Roush WR; Halterman RL J. Am. Chem. Soc 1986, 108, 294.
- Roush WR; Ando K; Powers DB; Palkowitz AD; Halterman RL J. Am. Chem. Soc 1990, 112, 6339.
- Seebach D; Beck AK; Imwinkelzied R; Roggo S; Wonnacott A Helv. Chim. Acta 1987, 70, 954.
- Riediker M; Duthaler RO Angew. Chem. Int. Ed 1989, 28, 494.
- Garcia J; Kim BM; Masamune SJ Org. Chem 1987, 52, 4831.
- Short RP; Masamune SJ Am. Chem. Soc 1989, 111, 1892.
- Reetz M Pure Appl. Chem 1988, 60, 1607.
- Corey EJ; Yu C-M; Kim SS J. Am. Chem. Soc 1989, 111, 5495.
- Kinnaird JWA; Ng PY; Kubota K; Wang X; Leighton JL J. Am. Chem. Soc 2002, 124, 7920. - PubMed
- Hackman BM; Lombardi PJ; Leighton JL Org. Lett 2004, 6, 4375. - PubMed
-
- Furuta K; Mouri M; Yamamoto H Synlett 1991, 561.
- Costa AL; Piazza MG; Tagliavini E; Trombini C; Umani-Ronchi AJ Am. Chem. Soc 1993, 115, 7001.
- Keck GE; Tarbet KH; Geraci LS J. Am. Chem. Soc 1993, 115, 8467.
- Denmark SE; Coe DM; Pratt NE; Griedel BD J. Org. Chem 1994, 59, 6161. - PubMed
- Denmark SE; Fu JJ Am. Chem. Soc 2001, 123, 9488. - PubMed
- Bandini M; Cozzi PG; Melchiorre P; Umani-Ronchi A Angew. Chem. Int. Ed 1999, 38, 3357. - PubMed
- Bandini M; Cozzi PG; Umani-Ronchi A Angew. Chem. Int. Ed 2000, 39, 2327. - PubMed
- Majdecki M; Niedbała P; Jurczak J ChemistrySelect 2020, 5, 6424.
- Majdecki M; Tyszka-Gumkowska A; Jurczak J Org. Lett 2020, 22, 8687. - PMC - PubMed
- Majdecki M; Grodek P; Jurczak JJ Org. Chem 2021, 86, 995. - PMC - PubMed
-
- For selected reviews on catalytic enantioselective carbonyl allylation and crotylation, see:
- Denmark SE; Fu J Chem. Rev 2003, 103, 2763. - PubMed
- Hall DG Synlett 2007, 1644.
- Hargaden GC; Guiry PJ Adv. Synth. Catal 2007, 349, 2407.
- Yus M; González-Gómez JC; Foubelo F Chem. Rev 2011, 111, 7774. - PubMed
- Huo H-X; Duvall JR; Huang M-Y; Hong R Org. Chem. Front 2014, 1, 303.
- Tian Q; Zhang G Synthesis 2016, 48, 4038.
- Spielmann K; Niel G; de Figueiredo RM; Campagne J-M Chem. Soc. Rev 2018, 47, 1159. - PubMed
- Rubtsov AE; Malkov Andrei V. Synlett 2021, 32, 1397.
- Neufeld J; Stünkel T; Mück-Lichtenfeld C; Daniliuc CG; Gilmour R Angew. Chem. Int. Ed 2021, in press; DOI: 10.1002/anie.202102222. - DOI - PMC - PubMed
-
- For selected reviews on polyketide total synthesis, see:
- Koskinen AMP; Karisalmi K Chem. Soc. Rev 2005, 34, 677. - PubMed
- Dechert-Schmitt A-MR; Schmitt DC; Gao X; Itoh T; Krische MJ Nat. Prod. Rep 2014, 31, 504. - PMC - PubMed
- Feng J; Kasun ZA; Krische MJ J. Am. Chem. Soc 2016, 138, 5467. - PMC - PubMed
- Liu H; Lin S; Jacobsen KM; Poulsen TB Angew. Chem. Int. Ed 2019, 58, 13630. - PubMed
- Doerksen RS; Meyer CC; Krische MJ Angew. Chem. Int. Ed 2019, 58, 14055. - PMC - PubMed
- Friedrich RM; Friestad GK Nat. Prod. Rep 2020, 37, 1229. - PubMed
- Sperandio C; Rodriguez J; Quintard A Org. Biomol. Chem 2020, 18, 1025. - PubMed
- He Y; Song H; Chen J; Zhu S Nat. Commun 2021, 12, 638; - PMC - PubMed
- Knochel P; Kremsmair A Synfacts 2021, 17, 0405.
Grants and funding
LinkOut - more resources
Full Text Sources
