Morphological, ultrastructural, genetic characteristics and remarkably low prevalence of macroscopic Sarcocystis species isolated from sheep and goats in Kurdistan region, Iraq
- PMID: 37841456
- PMCID: PMC10569315
- DOI: 10.3389/fvets.2023.1225796
Morphological, ultrastructural, genetic characteristics and remarkably low prevalence of macroscopic Sarcocystis species isolated from sheep and goats in Kurdistan region, Iraq
Abstract
Introduction: Sarcocystis is a genus of cyst-forming parasites that infest both humans and livestock. Some parasites cause clinical and subclinical diseases in their hosts, resulting in economic losses.
Methods: Esophagus, diaphragm, and skeletal muscle from slaughtered sheep and goats were examined macroscopically, microscopically, and ultrastructurally and subjected to DNA analysis.
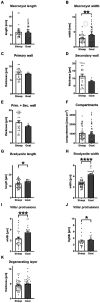
Results: We isolated macrocysts of S. gigantea and of S. caprafelis moulei from naturally infected sheep (Ovis aries) and goats (Capra hircus). The macrocyst wall thickness was 18.9 µm in sheep and 15.3 µm in goats, and consisted of an inner Periodic acid Schiff- (PAS) negative primary wall and an outer glycoconjugates containing i.e. PAS-positive secondary wall. The walls inner surface was compartmentalized and filled with bradyzoites. In S. gigantea the bradyzoites were approximently 12.3 x 2.6 µm in size, while in S. caprafelis moulei they were 13.9 x 4.4 µm. Ultrastructurally, both species have nearly identical morphology: cauliflower-like protrusions with numerous microtubules and often dendritic-like filaments, branching from the primary wall. The 18S rRNA gene in S. gigantea was 85.9% identical to that in S. medusiformis and 80.4% to the S. caprafelis moulei gene. The 28S rRNA gene in S. gigantea was 94.6% identical to that in S. medusiformis and 97.3% to the S. caprafelis moulei.
Conclusion: This study is the first to (i) detail the ultrastructure of the macrocyst wall of S. caprafelis moulei, (ii) identify S. medusiformis in Iraqi sheep, and (iii) compare the prevalence of macroscopic Sarcocystis at different time periods within the same region. A positive finding was the reduction of macroscopic sarcocystosis occurrences (0.01% in sheep and 0.02% in goats) compared to our previous data from 1992 (4.1%: sheep, 33.6%: goats).
Keywords: Sarcocystis gigantea; Sarcocystis medusiformis; Sarcocystis moulei; genetic analysis; goat; sheep.
Copyright © 2023 Nawshirwan, Heucken, Piekarek, van Beers, Fulgham-Scott, Grandoch, Neiss, Vogt and Barham.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
-
- Dubey JP, Carlero-Bernal R, Rosenthal BM, Speer CA, Fayer R. Sarcocystosis of animals and humans. 2nd ed. Boca Raton, FL: CRC Press; (2016).
-
- Prakas P, Bultkauslkas D, Rudaityte E, Kutkiene L, Srugosa A, Puraite I. Morphoogical and molecular characterization of Sarcocystis taeniata and Sarcocystis pilosa n. sp. from the sika deer (Cervus Nippon) in Lithuania. Parasitol Res. (2016) 115:3021–32. doi: 10.1007/s00436-016-5057-7, PMID: - DOI - PubMed
LinkOut - more resources
Full Text Sources

