Evidence For Cannabidiol Modulation of Serotonergic Transmission in a Model of Osteoarthritis via in vivo PET Imaging and Behavioral Assessment
- PMID: 37841504
- PMCID: PMC10576525
- DOI: 10.23958/ijirms/vol07-i06/1418
Evidence For Cannabidiol Modulation of Serotonergic Transmission in a Model of Osteoarthritis via in vivo PET Imaging and Behavioral Assessment
Abstract
Background: Preclinical studies indicate that cannabidiol (CBD), the primary nonaddictive component of cannabis, has a wide range of reported pharmacological effects such as analgesic and anxiolytic actions; however, the exact mechanisms of action for these effects have not been examined in chronic osteoarthritis (OA). Similar to other chronic pain syndromes, OA pain can have a significant affective component characterized by mood changes. Serotonin (5-HT) is a neurotransmitter implicated in pain, depression, and anxiety. Pain is often in comorbidity with mood and anxiety disorders in patients with OA. Since primary actions of CBD are analgesic and anxiolytic, in this first in vivo positron emission tomography (PET) imaging study, we investigate the interaction of CBD with serotonin 5-HT1A receptor via a combination of in vivo neuroimaging and behavioral studies in a well-validated OA animal model.
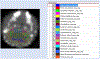
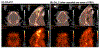
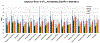
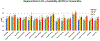
Methods: The first aim of this study was to evaluate the target involvement, including the evaluation of modulation by acute administration of CBD, or a specific target antagonist/agonist intervention, in control animals. The brain 5-HT1A activity/availability was assessed via in vivo dynamic PET imaging (up to 60 min) using a selective 5-HT1A radioligand ([18F]MeFWAY). Tracer bindings of 17 ROIs were evaluated based on averaged SUVR values over the last 10 min using CB as the reference region. We subsequently examined the neurochemical and behavioral alterations in OA animals (induction with monosodium iodoacetate (MIA) injection), as compared to control animals, via neuroimaging and behavioral assessment. Further, we examined the effects of repeated low-dose CBD treatment on mechanical allodynia (von Frey tests) and anxiety-like (light/dark box tests, L/D), depressive-like (forced swim tests, FST) behaviors in OA animals, as compared to after vehicle treatment.
Results: The tracer binding was significantly reduced in control animals after an acute dose of CBD administered intravenously (1.0 mg/kg, i.v.), as compared to that for baseline. This binding specificity to 5-HT1A was further confirmed by a similar reduction of tracer binding when a specific 5-HT1A antagonist WAY1006235 was used (0.3 mg/kg, i.v.). Mice subjected to the MIA-induced OA for 13-20 days showed a decreased 5-HT1A tracer binding (25% to 41%), consistent with the notion that 5-HT1A plays a role in the modulation of pain in OA. Repeated treatment with CBD administered subcutaneously (5 mg/kg/day, s.c., for 16 days after OA induction) increased 5-HT1A tracer binding, while no significant improvement was observed after vehicle. A trend of increased anxiety or depressive-like behavior in the light/dark box or forced swim tests after OA induction, and a decrease in those behaviors after repeated low-dose CBD treatment, are consistent with the anxiolytic action of CBD through 5HT1A receptor activation. There appeared to be a sex difference: females seem to be less responsive at the baseline towards pain stimuli, while being more sensitive to CBD treatment.
Conclusion: This first in vivo PET imaging study in an OA animal model has provided evidence for the interaction of CBD with the serotonin 5-HT1A receptor. Behavioral studies with more pharmacological interventions to support the target involvement are needed to further confirm these critical findings.
Keywords: Anxiety; Cannabidiol; Osteoarthritis; PET imaging; Pain; Serotonin receptor.
Conflict of interest statement
Competing interests: The authors declare that they have no competing interests.
Figures
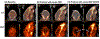
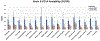
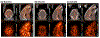



Similar articles
-
Cannabidiol modulates serotonergic transmission and reverses both allodynia and anxiety-like behavior in a model of neuropathic pain.Pain. 2019 Jan;160(1):136-150. doi: 10.1097/j.pain.0000000000001386. Pain. 2019. PMID: 30157131 Free PMC article.
-
Cannabidiol induces rapid-acting antidepressant-like effects and enhances cortical 5-HT/glutamate neurotransmission: role of 5-HT1A receptors.Neuropharmacology. 2016 Apr;103:16-26. doi: 10.1016/j.neuropharm.2015.12.017. Epub 2015 Dec 19. Neuropharmacology. 2016. PMID: 26711860
-
Motor effects of the non-psychotropic phytocannabinoid cannabidiol that are mediated by 5-HT1A receptors.Neuropharmacology. 2013 Dec;75:155-63. doi: 10.1016/j.neuropharm.2013.07.024. Epub 2013 Aug 4. Neuropharmacology. 2013. PMID: 23924692
-
Cannabidiol as a Potential Treatment for Anxiety and Mood Disorders: Molecular Targets and Epigenetic Insights from Preclinical Research.Int J Mol Sci. 2021 Feb 13;22(4):1863. doi: 10.3390/ijms22041863. Int J Mol Sci. 2021. PMID: 33668469 Free PMC article. Review.
-
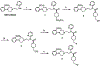
4-(2´-Methoxyphenyl)-1-[2´-(N-2´´-1,3 pyrimidino)-p-[18F]fluorobenzamido]ethylpiperazine.2005 Feb 1 [updated 2008 Jun 30]. In: Molecular Imaging and Contrast Agent Database (MICAD) [Internet]. Bethesda (MD): National Center for Biotechnology Information (US); 2004–2013. 2005 Feb 1 [updated 2008 Jun 30]. In: Molecular Imaging and Contrast Agent Database (MICAD) [Internet]. Bethesda (MD): National Center for Biotechnology Information (US); 2004–2013. PMID: 20641340 Free Books & Documents. Review.
References
-
- Achanath R, Jose J, Rangaswamy C, Mandal S, Kadivilpparampu AM, Newington IM, et al. (2015). Novel Synthesis Method.
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
