A Haspin-ARHGAP11A axis regulates epithelial morphogenesis through Rho-ROCK dependent modulation of LIMK1-Cofilin
- PMID: 37841592
- PMCID: PMC10570125
- DOI: 10.1016/j.isci.2023.108011
A Haspin-ARHGAP11A axis regulates epithelial morphogenesis through Rho-ROCK dependent modulation of LIMK1-Cofilin
Abstract
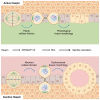
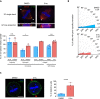
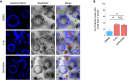
Throughout mitosis, a plethora of processes must be efficiently concerted to ensure cell proliferation and tissue functionality. The mitotic spindle does not only mediate chromosome segregation, but also defines the axis of cellular division, thus determining tissue morphology. Functional spindle orientation relies on precise actin dynamics, shaped in mitosis by the LIMK1-Cofilin axis. The kinase Haspin acts as a guardian of faithful chromosome segregation that ensures amphitelic chromosome attachment and prevents unscheduled cohesin cleavage. Here, we report an unprecedented role for Haspin in the determination of spindle orientation in mitosis. We show that, during mitosis, Haspin regulates Rho-ROCK activity through ARHGAP11A, a poorly characterized GAP, and that ROCK is in turn responsible for the mitotic activation of LIMK1 and stabilization of the actin cytoskeleton, thus supporting a functional spindle orientation. By exploiting 3D cell cultures, we show that this pathway is pivotal for the establishment of a morphologically functional tissue.
Keywords: Cell biology; Molecular biology.
© 2023 The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures



References
LinkOut - more resources
Full Text Sources
Miscellaneous

