FZL, a dynamin-like protein localized to curved grana edges, is required for efficient photosynthetic electron transfer in Arabidopsis
- PMID: 37841601
- PMCID: PMC10568140
- DOI: 10.3389/fpls.2023.1279699
FZL, a dynamin-like protein localized to curved grana edges, is required for efficient photosynthetic electron transfer in Arabidopsis
Abstract
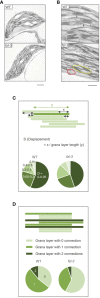
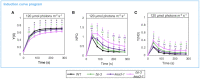
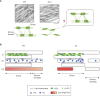
Photosynthetic electron transfer and its regulation processes take place on thylakoid membranes, and the thylakoid of vascular plants exhibits particularly intricate structure consisting of stacked grana and flat stroma lamellae. It is known that several membrane remodeling proteins contribute to maintain the thylakoid structure, and one putative example is FUZZY ONION LIKE (FZL). In this study, we re-evaluated the controversial function of FZL in thylakoid membrane remodeling and in photosynthesis. We investigated the sub-membrane localization of FZL and found that it is enriched on curved grana edges of thylakoid membranes, consistent with the previously proposed model that FZL mediates fusion of grana and stroma lamellae at the interfaces. The mature fzl thylakoid morphology characterized with the staggered and less connected grana seems to agree with this model as well. In the photosynthetic analysis, the fzl knockout mutants in Arabidopsis displayed reduced electron flow, likely resulting in higher oxidative levels of Photosystem I (PSI) and smaller proton motive force (pmf). However, nonphotochemical quenching (NPQ) of chlorophyll fluorescence was excessively enhanced considering the pmf levels in fzl, and we found that introducing kea3-1 mutation, lowering pH in thylakoid lumen, synergistically reinforced the photosynthetic disorder in the fzl mutant background. We also showed that state transitions normally occurred in fzl, and that they were not involved in the photosynthetic disorders in fzl. We discuss the possible mechanisms by which the altered thylakoid morphology in fzl leads to the photosynthetic modifications.
Keywords: Arabidopsis; FUZZY ONION LIKE (FZL); chloroplast; photosynthetic electron transfer; thylakoid; thylakoid structure.
Copyright © 2023 Ogawa, Iwano, Shikanai and Sakamoto.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures





Similar articles
-
FZL, an FZO-like protein in plants, is a determinant of thylakoid and chloroplast morphology.Proc Natl Acad Sci U S A. 2006 Apr 25;103(17):6759-64. doi: 10.1073/pnas.0507287103. Epub 2006 Apr 14. Proc Natl Acad Sci U S A. 2006. PMID: 16617119 Free PMC article.
-
FZL is primarily localized to the inner chloroplast membrane however influences thylakoid maintenance.Plant Mol Biol. 2018 Jul;97(4-5):421-433. doi: 10.1007/s11103-018-0748-3. Epub 2018 Jun 27. Plant Mol Biol. 2018. PMID: 29951988
-
Thylakoid-Bound Polysomes and a Dynamin-Related Protein, FZL, Mediate Critical Stages of the Linear Chloroplast Biogenesis Program in Greening Arabidopsis Cotyledons.Plant Cell. 2018 Jul;30(7):1476-1495. doi: 10.1105/tpc.17.00972. Epub 2018 Jun 7. Plant Cell. 2018. PMID: 29880711 Free PMC article.
-
Contribution of Cyclic and Pseudo-cyclic Electron Transport to the Formation of Proton Motive Force in Chloroplasts.Mol Plant. 2017 Jan 9;10(1):20-29. doi: 10.1016/j.molp.2016.08.004. Epub 2016 Aug 26. Mol Plant. 2017. PMID: 27575692 Review.
-
Dynamic flexibility in the structure and function of photosystem II in higher plant thylakoid membranes: the grana enigma.Photosynth Res. 2008 Oct-Dec;98(1-3):575-87. doi: 10.1007/s11120-008-9381-3. Epub 2008 Nov 8. Photosynth Res. 2008. PMID: 18998237 Review.
Cited by
-
Regulation of Microalgal Photosynthetic Electron Transfer.Plants (Basel). 2024 Jul 29;13(15):2103. doi: 10.3390/plants13152103. Plants (Basel). 2024. PMID: 39124221 Free PMC article. Review.
-
Localization of proteins involved in the biogenesis and repair of the photosynthetic apparatus to thylakoid subdomains in Arabidopsis.Plant Direct. 2024 Nov 13;8(11):e70008. doi: 10.1002/pld3.70008. eCollection 2024 Nov. Plant Direct. 2024. PMID: 39544483 Free PMC article.
References
-
- Amann K., Lezhneva L., Wanner G., Herrmann R. G., Meurer J. (2004). ACCUMULATION OF PHOTOSYSTEM ONE1, a member of a novel gene family, is required for accumulation of [4Fe-4S] cluster-containing chloroplast complexes and antenna proteins. Plant Cell 16, 3084–3097. doi: 10.1105/tpc.104.024935 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources

