A BAHD acyltransferase contributes to the biosynthesis of both ethyl benzoate and methyl benzoate in the flowers of Lilium oriental hybrid 'Siberia'
- PMID: 37841617
- PMCID: PMC10570747
- DOI: 10.3389/fpls.2023.1275960
A BAHD acyltransferase contributes to the biosynthesis of both ethyl benzoate and methyl benzoate in the flowers of Lilium oriental hybrid 'Siberia'
Abstract
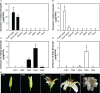
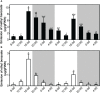
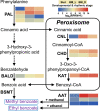
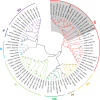
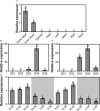
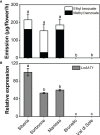
Lily is a popular flower worldwide due to its elegant appearance and pleasant fragrance. Floral volatiles of lily are predominated by monoterpenes and benzenoids. While a number of genes for monoterpene biosynthesis have been characterized, the molecular mechanism underlying floral benzenoid formation in lily remains unclear. Here, we report on the identification and characterization of a novel BAHD acyltransferase gene that contributes to the biosynthesis of two related floral scent benzoate esters, ethyl benzoate and methyl benzoate, in the scented Lilium oriental hybrid 'Siberia'. The emission of both methyl benzoate and ethyl benzoate in L. 'Siberia' was found to be tepal-specific, floral development-regulated and rhythmic. Through transcriptome profiling and bioinformatic analysis, a BAHD acyltransferase gene designated LoAAT1 was identified as the top candidate gene for the production of ethyl benzoate. In vitro enzyme assays and substrate feeding assays provide substantial evidence that LoAAT1 is responsible for the biosynthesis of ethyl benzoate. It was interesting to note that in in vitro enzyme assay, LoAAT1 can also catalyze the formation of methyl benzoate, which is typically formed by the action of benzoic acid methyltransferase (BAMT). The lack of an expressed putative BAMT gene in the flower transcriptome of L. 'Siberia', together with biochemical and expression evidence, led us to conclude that LoAAT1 is also responsible for, or at least contributes to, the biosynthesis of the floral scent compound methyl benzoate. This is the first report that a member of the plant BAHD acyltransferase family contributes to the production of both ethyl benzoate and methyl benzoate, presenting a new mechanism for the biosynthesis of benzoate esters.
Keywords: BAHD acyltransferase; biosynthesis; ethyl benzoate; floral scent; lily; methyl benzoate.
Copyright © 2023 Yue, Wang, Li, Liu, Yin, Huang, Zhou, Li, Yu, Chen, Yu and Fan.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Figures

Similar articles
-
Transcriptome Sequencing Analysis Reveals a Difference in Monoterpene Biosynthesis between Scented Lilium 'Siberia' and Unscented Lilium 'Novano'.Front Plant Sci. 2017 Aug 4;8:1351. doi: 10.3389/fpls.2017.01351. eCollection 2017. Front Plant Sci. 2017. PMID: 28824685 Free PMC article.
-
Functional characterization and expression analysis of two terpene synthases involved in floral scent formation in Lilium 'Siberia'.Planta. 2019 Jan;249(1):71-93. doi: 10.1007/s00425-018-3006-7. Epub 2018 Sep 14. Planta. 2019. PMID: 30218384
-
LiNAC100 contributes to linalool biosynthesis by directly regulating LiLiS in Lilium 'Siberia'.Planta. 2024 Feb 23;259(4):73. doi: 10.1007/s00425-024-04340-2. Planta. 2024. PMID: 38393405
-
Floral benzenoid carboxyl methyltransferases: from in vitro to in planta function.Phytochemistry. 2005 Jun;66(11):1211-30. doi: 10.1016/j.phytochem.2005.03.031. Phytochemistry. 2005. PMID: 15946712 Free PMC article. Review.
-
Genetic aspects of floral fragrance in plants.Biochemistry (Mosc). 2007 Apr;72(4):351-8. doi: 10.1134/s0006297907040013. Biochemistry (Mosc). 2007. PMID: 17511599 Review.
Cited by
-
Genome-wide identification of tea plant (Camellia sinensis) BAHD acyltransferases reveals their role in response to herbivorous pests.BMC Plant Biol. 2024 Apr 1;24(1):229. doi: 10.1186/s12870-024-04867-2. BMC Plant Biol. 2024. PMID: 38561653 Free PMC article.
-
The Scent of Lily Flowers: Advances in the Identification, Biosynthesis, and Regulation of Fragrance Components.Int J Mol Sci. 2025 Jan 8;26(2):468. doi: 10.3390/ijms26020468. Int J Mol Sci. 2025. PMID: 39859184 Free PMC article. Review.
-
Determination of Volatile Organic Compounds and Endogenous Extracts and Study of Expression Patterns of TPS and BSMT in the Flowers of Seven Lilium Cultivars.Molecules. 2023 Dec 5;28(24):7938. doi: 10.3390/molecules28247938. Molecules. 2023. PMID: 38138428 Free PMC article.
-
Identification and Characterization of Jasmonic Acid Methyltransferase Involved in the Formation of Floral Methyl Jasmonate in Hedychium coronarium.Plants (Basel). 2023 Dec 19;13(1):8. doi: 10.3390/plants13010008. Plants (Basel). 2023. PMID: 38202316 Free PMC article.
-
Methylation Modification in Ornamental Plants: Impact on Floral Aroma and Color.Int J Mol Sci. 2024 Jul 29;25(15):8267. doi: 10.3390/ijms25158267. Int J Mol Sci. 2024. PMID: 39125834 Free PMC article. Review.
References
-
- Colquhoun T. A., Marciniak D. M., Wedde A. E., Kim J. Y., Schwieterman M. L., Levin L. A., et al. . (2012). A peroxisomally localized acyl-activating enzyme is required for volatile benzenoid formation in a Petunia×hybrida cv. 'Mitchell Diploid' flower. J. Exp. Bot. 63, 4821–4833. doi: 10.1093/jxb/ers153 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources

