Mechanobiology of portal hypertension
- PMID: 37841641
- PMCID: PMC10568428
- DOI: 10.1016/j.jhepr.2023.100869
Mechanobiology of portal hypertension
Abstract
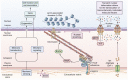
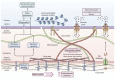
The interplay between mechanical stimuli and cellular mechanobiology orchestrates the physiology of tissues and organs in a dynamic balance characterized by constant remodelling and adaptative processes. Environmental mechanical properties can be interpreted as a complex set of information and instructions that cells read continuously, and to which they respond. In cirrhosis, chronic inflammation and injury drive liver cells dysfunction, leading to excessive extracellular matrix deposition, sinusoidal pseudocapillarization, vascular occlusion and parenchymal extinction. These pathological events result in marked remodelling of the liver microarchitecture, which is cause and result of abnormal environmental mechanical forces, triggering and sustaining the long-standing and progressive process of liver fibrosis. Multiple mechanical forces such as strain, shear stress, and hydrostatic pressure can converge at different stages of the disease until reaching a point of no return where the fibrosis is considered non-reversible. Thereafter, reciprocal communication between cells and their niches becomes the driving force for disease progression. Accumulating evidence supports the idea that, rather than being a passive consequence of fibrosis and portal hypertension (PH), mechanical force-mediated pathways could themselves represent strategic targets for novel therapeutic approaches. In this manuscript, we aim to provide a comprehensive review of the mechanobiology of PH, by furnishing an introduction on the most important mechanisms, integrating these concepts into a discussion on the pathogenesis of PH, and exploring potential therapeutic strategies.
Keywords: HSC; LSEC; Liver cirrhosis; hepatic stellate cells; liver fibrosis; liver sinusoidal endothelial cells.
© 2023 The Author(s).
Conflict of interest statement
The authors declare no conflict of interest. Please refer to the accompanying ICMJE disclosure forms for further details.
Figures

Similar articles
-
Cooperation of liver cells in health and disease.Adv Anat Embryol Cell Biol. 2001;161:III-XIII, 1-151. doi: 10.1007/978-3-642-56553-3. Adv Anat Embryol Cell Biol. 2001. PMID: 11729749 Review.
-
Increased sinusoidal pressure impairs liver endothelial mechanosensing, uncovering novel biomarkers of portal hypertension.JHEP Rep. 2023 Mar 8;5(6):100722. doi: 10.1016/j.jhepr.2023.100722. eCollection 2023 Jun. JHEP Rep. 2023. PMID: 37151732 Free PMC article.
-
Pathophysiology and a Rational Basis of Therapy.Dig Dis. 2015;33(4):508-14. doi: 10.1159/000374099. Epub 2015 Jul 6. Dig Dis. 2015. PMID: 26159267
-
Pan-PPAR agonist lanifibranor improves portal hypertension and hepatic fibrosis in experimental advanced chronic liver disease.J Hepatol. 2021 May;74(5):1188-1199. doi: 10.1016/j.jhep.2020.11.045. Epub 2020 Dec 2. J Hepatol. 2021. PMID: 33278455
-
Liver fibrosis: from the bench to clinical targets.Dig Liver Dis. 2004 Apr;36(4):231-42. doi: 10.1016/j.dld.2004.01.003. Dig Liver Dis. 2004. PMID: 15115333 Review.
Cited by
-
Sinusoidal communication in chronic liver disease.Clin Mol Hepatol. 2025 Jan;31(1):32-55. doi: 10.3350/cmh.2024.0734. Epub 2024 Oct 2. Clin Mol Hepatol. 2025. PMID: 39355871 Free PMC article. Review.
-
Semiconducting polymer dots for multifunctional integrated nanomedicine carriers.Mater Today Bio. 2024 Mar 24;26:101028. doi: 10.1016/j.mtbio.2024.101028. eCollection 2024 Jun. Mater Today Bio. 2024. PMID: 38590985 Free PMC article. Review.
-
Mechanisms and implications of recompensation in cirrhosis.JHEP Rep. 2024 Oct 10;6(12):101233. doi: 10.1016/j.jhepr.2024.101233. eCollection 2024 Dec. JHEP Rep. 2024. PMID: 39640222 Free PMC article. Review.
-
Vasomics of the liver.Gut. 2025 May 7;74(6):1008-1020. doi: 10.1136/gutjnl-2024-334133. Gut. 2025. PMID: 40044498 Free PMC article. Review.
-
Recent advances in cancer detection using dynamic, stimuli-responsive supramolecular chemosensors. a focus review.Front Chem. 2024 Oct 7;12:1478034. doi: 10.3389/fchem.2024.1478034. eCollection 2024. Front Chem. 2024. PMID: 39435264 Free PMC article. Review.
References
-
- Dupont S., Wickstrom S.A. Mechanical regulation of chromatin and transcription. Nat Rev Genet. 2022;23:624–643. - PubMed
-
- Gracia-Sancho J., Caparros E., Fernandez-Iglesias A., Frances R. Role of liver sinusoidal endothelial cells in liver diseases. Nat Rev Gastroenterol Hepatol. 2021;18:411–431. - PubMed
-
- Baffy G. Origins of portal hypertension in nonalcoholic fatty liver disease. Dig Dis Sci. 2018;63:563–576. - PubMed

