Case report: Chronic pain in a pediatric patient with late-onset pompe disease
- PMID: 37841659
- PMCID: PMC10575478
- DOI: 10.3389/fpain.2023.1244609
Case report: Chronic pain in a pediatric patient with late-onset pompe disease
Abstract
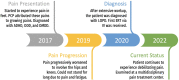
Pompe disease (PD) is a rare inherited metabolic disorder of deficient or absent acid alpha-glucosidase (GAA), resulting in defective lysosomal glycogen catabolism. Muscle weakness, respiratory deficiency and gastrointestinal symptoms are commonly monitored in PD. However, pain and associated psychological symptoms are less focused upon. A pediatric patient with late-onset Pompe disease (LOPD) comorbid with chronic pain is presented. Symptoms of pain in the feet were first reported between 6 and 7 years of age and were attributed to growing pains. Following progression of lower body pain, weakness, fatigue, and difficulties with ambulation, a thorough clinical assessment including genetic testing was performed, which led to a diagnosis of LOPD at 9 years of age. ERT with recombinant human alglucosidase alfa was subsequently started. The patient's clinical status is compounded by depressed mood, anxiety, and attention deficit hyperactivity disorder, which may further exacerbate pain. A multidisciplinary pain treatment approach consisting of orthopedics, physical therapy, and psychosocial therapy aimed at enhancing pain coping skills is described for this LOPD patient. This case highlights the need for a greater understanding of pain generation and identification of optimized pain treatment approaches in children with LOPD that can be implemented alongside ERT.
Keywords: ADHD; analgesia; depression; lysosomal storage disease; pain; pompe disease.
© 2023 Cao, van Gool, Golden, Goodlett, Camelo, Bujoreanu, Al-Hertani and Upadhyay.
Conflict of interest statement
JU and WA receive funding support from SanofiGenzyme. WA is a site Principal Investigator for the Early Access Program with Arimoclomol in US Patients with NPC (NCT04316637), which is sponsored by KemPharm Denmark A/S WA is on the advisory board for Beam Therapeutics. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
-
- Reuser AJJ, Hirschhorn R, Kroos MA. Pompe disease: glycogen storage disease type II, acid α-glucosidase (acid maltase) deficiency. In: Valle DL, Antonarakis S, Ballabio A, Beaudet AL, Mitchell GA, editors. The online metabolic and molecular bases of inherited disease. New York, NY: McGraw-Hill Education; (2019).
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

