Raman spectroscopy to assess the differentiation of bone marrow mesenchymal stem cells into a glial phenotype
- PMID: 37841662
- PMCID: PMC10570561
- DOI: 10.1016/j.reth.2023.09.016
Raman spectroscopy to assess the differentiation of bone marrow mesenchymal stem cells into a glial phenotype
Abstract
Background: Mesenchymal stem cells (MSCs) are multipotent precursor cells with the ability to self-renew and differentiate into multiple cell linage, including the Schwann-like fate that promotes regeneration after lesion. Raman spectroscopy provides a precise characterization of the osteogenic, adipogenic, hepatogenic and myogenic differentiation of MSCs. However, the differentiation of bone marrow mesenchymal stem cells (BMSCs) towards a glial phenotype (Schwann-like cells) has not been characterized before using Raman spectroscopy.
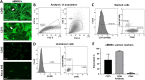
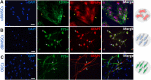
Method: We evaluated three conditions: 1) cell culture from rat bone marrow undifferentiated (uBMSCs), and two conditions of differentiation; 2) cells exposed to olfactory ensheathing cells-conditioned medium (dBMSCs) and 3) cells obtained from olfactory bulb (OECs). uBMSCs phenotyping was confirmed by morphology, immunocytochemistry and flow cytometry using antibodies of cell surface: CD90 and CD73. Glial phenotype of dBMSCs and OECs were verified by morphology and immunocytochemistry using markers of Schwann-like cells and OECs such as GFAP, p75 NTR and O4. Then, the Principal Component Analysis (PCA) of Raman spectroscopy was performed to discriminate components from the high wavenumber region between undifferentiated and glial-differentiated cells. Raman bands at the fingerprint region also were used to analyze the differentiation between conditions.
Results: Differences between Raman spectra from uBMSC and glial phenotype groups were noted at multiple Raman shift values. A significant decrease in the concentration of all major cellular components, including nucleic acids, proteins, and lipids were found in the glial phenotype groups. PCA analysis confirmed that the highest spectral variations between groups came from the high wavenumber region observed in undifferentiated cells and contributed with the discrimination between glial phenotype groups.
Conclusion: These findings support the use of Raman spectroscopy for the characterization of uBMSCs and its differentiation in the glial phenotype.
Keywords: Cell differentiation; Glial phenotype; Mesenchymal stem cells; Raman spectroscopy; conditioned medium.
© 2023 The Japanese Society for Regenerative Medicine. Production and hosting by Elsevier B.V.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




Similar articles
-
GFAPβ and GFAPδ Isoforms Expression in Mesenchymal Stem Cells, MSCs Differentiated Towards Schwann-like, and Olfactory Ensheathing Cells.Curr Issues Mol Biol. 2025 Jan 9;47(1):35. doi: 10.3390/cimb47010035. Curr Issues Mol Biol. 2025. PMID: 39852150 Free PMC article.
-
Cell surface expression of 27C7 by neonatal rat olfactory ensheathing cells in situ and in vitro is independent of axonal contact.Histochem Cell Biol. 2011 Apr;135(4):397-408. doi: 10.1007/s00418-011-0796-0. Epub 2011 Mar 10. Histochem Cell Biol. 2011. PMID: 21437623
-
In Vitro Transdifferentiation Potential of Equine Mesenchymal Stem Cells into Schwann-Like Cells.Stem Cells Dev. 2023 Jul;32(13-14):422-432. doi: 10.1089/scd.2022.0274. Epub 2023 Jun 7. Stem Cells Dev. 2023. PMID: 37071193 Free PMC article.
-
A Single-Cell Raman Spectroscopy Analysis of Bone Marrow Mesenchymal Stem/Stromal Cells to Identify Inter-Individual Diversity.Int J Mol Sci. 2022 Apr 28;23(9):4915. doi: 10.3390/ijms23094915. Int J Mol Sci. 2022. PMID: 35563306 Free PMC article.
-
[Phenotypic plasticity of neural crest-derived melanocytes and Schwann cells].Biol Aujourdhui. 2011;205(1):53-61. doi: 10.1051/jbio/2011008. Epub 2011 Apr 19. Biol Aujourdhui. 2011. PMID: 21501576 Review. French.
Cited by
-
GFAPβ and GFAPδ Isoforms Expression in Mesenchymal Stem Cells, MSCs Differentiated Towards Schwann-like, and Olfactory Ensheathing Cells.Curr Issues Mol Biol. 2025 Jan 9;47(1):35. doi: 10.3390/cimb47010035. Curr Issues Mol Biol. 2025. PMID: 39852150 Free PMC article.
References
-
- Kobolak J., Dinnyes A., Memic A., Khademhosseini A., Mobasheri A. Mesenchymal stem cells: identification, phenotypic characterization, biological properties and potential for regenerative medicine through biomaterial micro-engineering of their niche. Methods. 2016;99:62–68. doi: 10.1016/j.ymeth.2015.09.016. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

