Fecal microbial transplantation limits neural injury severity and functional deficits in a pediatric piglet traumatic brain injury model
- PMID: 37841685
- PMCID: PMC10568032
- DOI: 10.3389/fnins.2023.1249539
Fecal microbial transplantation limits neural injury severity and functional deficits in a pediatric piglet traumatic brain injury model
Abstract
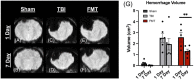
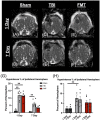
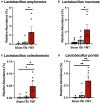
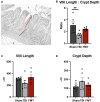
Pediatric traumatic brain injury (TBI) is a leading cause of death and disability in children. Due to bidirectional communication between the brain and gut microbial population, introduction of key gut bacteria may mitigate critical TBI-induced secondary injury cascades, thus lessening neural damage and improving functional outcomes. The objective of this study was to determine the efficacy of a daily fecal microbial transplant (FMT) to alleviate neural injury severity, prevent gut dysbiosis, and improve functional recovery post TBI in a translational pediatric piglet model. Male piglets at 4-weeks of age were randomly assigned to Sham + saline, TBI + saline, or TBI + FMT treatment groups. A moderate/severe TBI was induced by controlled cortical impact and Sham pigs underwent craniectomy surgery only. FMT or saline were administered by oral gavage daily for 7 days. MRI was performed 1 day (1D) and 7 days (7D) post TBI. Fecal and cecal samples were collected for 16S rRNA gene sequencing. Ipsilateral brain and ileum tissue samples were collected for histological assessment. Gait and behavior testing were conducted at multiple timepoints. MRI showed that FMT treated animals demonstrated decreased lesion volume and hemorrhage volume at 7D post TBI as compared to 1D post TBI. Histological analysis revealed improved neuron and oligodendrocyte survival and restored ileum tissue morphology at 7D post TBI in FMT treated animals. Microbiome analysis indicated decreased dysbiosis in FMT treated animals with an increase in multiple probiotic Lactobacilli species, associated with anti-inflammatory therapeutic effects, in the cecum of the FMT treated animals, while non-treated TBI animals showed an increase in pathogenic bacteria, associated with inflammation and disease such in feces. FMT mediated enhanced cellular and tissue recovery resulted in improved motor function including stride and step length and voluntary motor activity in FMT treated animals. Here we report for the first time in a highly translatable pediatric piglet TBI model, the potential of FMT treatment to significantly limit cellular and tissue damage leading to improved functional outcomes following a TBI.
Keywords: MRI; behavior analysis; fecal matter transfer; gait analysis; microbiome gut-brain axis; porcine (pig) model; traumatic brain injury.
Copyright © 2023 Fagan, Welch, Scheulin, Sneed, Jeon, Golan, Cheek, Barany, Oeltzschner, Callaway, Zhao, Park, Lourenco, Duberstein and West.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures




Similar articles
-
Fecal Microbiota Transfer Attenuates Gut Dysbiosis and Functional Deficits After Traumatic Brain Injury.Shock. 2022 Jun 1;57(6):251-259. doi: 10.1097/SHK.0000000000001934. Shock. 2022. PMID: 35759305 Free PMC article.
-
POSTINJURY FECAL MICROBIOME TRANSPLANT DECREASES LESION SIZE AND NEUROINFLAMMATION IN TRAUMATIC BRAIN INJURY.Shock. 2022 Oct 1;58(4):287-294. doi: 10.1097/SHK.0000000000001979. Epub 2022 Aug 18. Shock. 2022. PMID: 36256625 Free PMC article.
-
Fecal Microbiota Transplantation Is a Promising Method to Restore Gut Microbiota Dysbiosis and Relieve Neurological Deficits after Traumatic Brain Injury.Oxid Med Cell Longev. 2021 Feb 10;2021:5816837. doi: 10.1155/2021/5816837. eCollection 2021. Oxid Med Cell Longev. 2021. PMID: 33628361 Free PMC article.
-
Fecal transplantation for treatment of inflammatory bowel disease.Cochrane Database Syst Rev. 2018 Nov 13;11(11):CD012774. doi: 10.1002/14651858.CD012774.pub2. Cochrane Database Syst Rev. 2018. Update in: Cochrane Database Syst Rev. 2023 Apr 25;4:CD012774. doi: 10.1002/14651858.CD012774.pub3. PMID: 30480772 Free PMC article. Updated.
-
Research progress on the relationship between traumatic brain injury and brain-gut-microbial axis.Ibrain. 2024 Mar 30;10(4):477-487. doi: 10.1002/ibra.12153. eCollection 2024 Winter. Ibrain. 2024. PMID: 39691426 Free PMC article. Review.
Cited by
-
Cognitive Function and the Consumption of Probiotic Foods: A National Health and Nutrition Examination Survey Study.Nutrients. 2024 Oct 25;16(21):3631. doi: 10.3390/nu16213631. Nutrients. 2024. PMID: 39519464 Free PMC article.
-
Catalase Activity in the Brain Is Associated with Recovery from Brain Injury in a Piglet Model of Traumatic Brain Injury.Brain Sci. 2025 Jun 4;15(6):608. doi: 10.3390/brainsci15060608. Brain Sci. 2025. PMID: 40563779 Free PMC article.
-
White Matter Integrity and Motor Function Disruption Due to Traumatic Brain Injury in Piglets: Impacts on Motor-Related Brain Fibers.Brain Sci. 2024 Mar 2;14(3):247. doi: 10.3390/brainsci14030247. Brain Sci. 2024. PMID: 38539635 Free PMC article.
-
Human-Induced Pluripotent Stem Cell-Derived Neural Stem Cell Therapy Limits Tissue Damage and Promotes Tissue Regeneration and Functional Recovery in a Pediatric Piglet Traumatic-Brain-Injury Model.Biomedicines. 2024 Jul 25;12(8):1663. doi: 10.3390/biomedicines12081663. Biomedicines. 2024. PMID: 39200128 Free PMC article.
References
Associated data
LinkOut - more resources
Full Text Sources

