This is a preprint.
Prophylactic and long-lasting efficacy of senolytic CAR T cells against age-related metabolic dysfunction
- PMID: 37841853
- PMCID: PMC10571605
- DOI: 10.21203/rs.3.rs-3385749/v1
Prophylactic and long-lasting efficacy of senolytic CAR T cells against age-related metabolic dysfunction
Update in
-
Prophylactic and long-lasting efficacy of senolytic CAR T cells against age-related metabolic dysfunction.Nat Aging. 2024 Mar;4(3):336-349. doi: 10.1038/s43587-023-00560-5. Epub 2024 Jan 24. Nat Aging. 2024. PMID: 38267706 Free PMC article.
Abstract
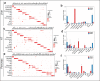
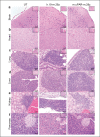
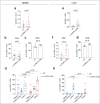
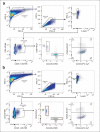
Senescent cells accumulate in organisms over time because of tissue damage and impaired immune surveillance and contribute to age-related tissue decline1,2. In agreement, genetic ablation studies reveal that elimination of senescent cells from aged tissues can ameliorate various age-related pathologies, including metabolic dysfunction and decreased physical fitness3-7. While small-molecule drugs capable of eliminating senescent cells (known as 'senolytics') partially replicate these phenotypes, many have undefined mechanisms of action and all require continuous administration to be effective. As an alternative approach, we have developed a cell-based senolytic therapy based on chimeric antigen receptor (CAR) T cells targeting uPAR, a cell-surface protein upregulated on senescent cells, and previously showed these can safely and efficiently eliminate senescent cells in young animals and reverse liver fibrosis8. We now show that uPAR-positive senescent cells accumulate during physiological aging and that they can be safely targeted with senolytic CAR T cells. Treatment with anti uPAR CAR T cells ameliorates metabolic dysfunction by improving glucose tolerance and exercise capacity in physiological aging as well as in a model of metabolic syndrome. Importantly, a single administration of a low dose of these senolytic CAR T cells is sufficient to achieve long-term therapeutic and preventive effects.
Conflict of interest statement
Competing Interests C.A., J.F., M.S. and S.W.L. are listed as the inventors of several patent applications (62/800,188; 63/174,277; 63/209,941; 63/209,940; 63/209,915; 63/209,924; 17/426,728; 3,128,368; 20748891.7; 2020216486) related to senolytic CAR T cells. C.A., M.S. and S.W.L. are advisors for Fate Therapeutics. S.W.L. also has provided consultancy for and had equity in Oric Pharmaceuticals, Blueprint Medicines, Mirimus Inc, Senecea Therapeutics, Faeth Therapeutics, and PMV Pharmaceuticals. D.A.T. is a member of the Scientific Advisory Board and receives stock options from Leap Therapeutics, Dunad Therapeutics, Cygnal Therapeutics and Mestag Therapeutics outside the submitted work. D.A.T. is scientific co-founder of Mestag Therapeutics. D.A.T. has received research grant support from Fibrogen, Mestag, and ONO Therapeutics. D.A.T. receives grant funding from the Lustgarten Foundation, the NIH, and the Thompson Foundation. None of this work is related to the publication. No other disclosures were reported. R.L.L. is on the supervisory board of Qiagen and is a scientific advisor to Imago, Mission Bio, Zentalis, Ajax, Auron, Prelude, C4 Therapeutics and Isoplexis. R.L.L. receives research support from Ajax, Zentalis and Abbvie and has consulted for Incyte, Janssen and Astra Zeneca and has received honoraria from Astra Zeneca for invited lectures. L.W.J owns stock in Pacyclex, Inc., and Illuminosonics, Inc. M.S. holds other unrelated patents on CAR technologies. S.W.L is an advisor for and has equity in the following biotechnology companies: ORIC Pharmaceuticals, Faeth Therapeutics, Blueprint Medicines, Geras Bio, Mirimus Inc., PMV Pharmaceuticals and Constellation Pharmaceuticals.
Figures












References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

