Methylene blue in anticancer photodynamic therapy: systematic review of preclinical studies
- PMID: 37841915
- PMCID: PMC10568458
- DOI: 10.3389/fphar.2023.1264961
Methylene blue in anticancer photodynamic therapy: systematic review of preclinical studies
Abstract
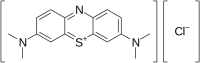
Background: Methylene blue has a long history of clinical application. Thanks to phenothiazine chromophore, it has potential in photodynamic anticancer therapy. In spite of the growing body of literature that has evaluated the action of this dye on different types of cancer, the systematic understanding of this problem is still lacking. Therefore, this systematic review was performed to study the efficacy of methylene blue in photodynamic anticancer therapy. Methods: This systematic review was carried out in accordance with the PRISMA guidelines, and the study protocol was registered in PROSPERO (CRD42022368738). Articles for the systematic review were identified through the PubMed database. SYRCLE's risk of bias tool was used to assess the studies. The results of systematic analysis are presented as narrative synthesis. Results: Ten studies met the inclusion criteria and these full texts were reviewed. In the selected articles, the dosage of dye infusion ranged from 0.04 to 24.12 mg/kg. The effectiveness of photodynamic therapy with methylene blue against different types of cancer was confirmed by a decrease in tumor sizes in seven articles. Conclusion: The results of the systematic review support the suggestions that photodynamic therapy with methylene blue helps against different types of cancer, including colorectal tumor, carcinoma, and melanoma. In cases of nanopharmaceutics use, a considerable increase of anticancer therapy effectiveness was observed. The further research into methylene blue in photodynamic anticancer therapy is needed. Systematic Review Registration: (https://www.crd.york.ac.uk/prospero/display_record.php?RecordID=368738), identifier (CRD42022368738).
Keywords: cancer; methylene blue; nanopharmaceutics; photodynamic therapy; systematic review; tumor.
Copyright © 2023 Taldaev, Terekhov, Nikitin, Melnik, Kuzina, Klochko, Reshetov, Shiryaev, Loschenov and Ramenskaya.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
-
- Avdeeva Z. I., Soldatov A. A., Alpatova N. A., Medunicyn N. V., Bondarev V. P., Mironov A. N., et al. (2015). New generation monoclonal antibody drugs (problems and prospects). Bioprep. Profil. Diagn. Lečenie. 53 (1), 21–35.
-
- Cecatto R. B., de Magalhaes L. S., Rodrigues M. F. S. D., Pavani C., Lino-dos-Santos-Franco A., Gomes M. T., et al. (2020). Methylene blue mediated antimicrobial photodynamic therapy in clinical human studies: the state of the art. Photodiagnosis Photodyn. Ther. 31, 101828. 10.1016/j.pdpdt.2020.101828 - DOI - PubMed
-
- Dos Santos M. S. C., Gouvêa A. L., de Moura L. D., Paterno L. G., de Souza P. E. N., Bastos A. P., et al. (2018). Nanographene oxide-methylene blue as phototherapies platform for breast tumor ablation and metastasis prevention in a syngeneic orthotopic murine model. J. Nanobiotechnology. 16 (1), 9. 10.1186/s12951-018-0333-6 - DOI - PMC - PubMed
-
- Ehrlich P., Leppmann A. (1890). Ueber schmerzstillende wirkung des methylenblau. Dtsch. Med. Wochenschr. 16 (23), 493–494. 10.1055/s-0029-1209911 - DOI
-
- Ehrlich P. (1886). Ueber die Methylenblaureaction der lebenden Nervensubstanz. Dtsch. Med. Wochenschr. 12 (04), 49–52. 10.1055/s-0028-1139684 - DOI
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous