A review of the pathophysiology and the role of ion channels on bronchial asthma
- PMID: 37841931
- PMCID: PMC10568497
- DOI: 10.3389/fphar.2023.1236550
A review of the pathophysiology and the role of ion channels on bronchial asthma
Abstract
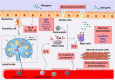
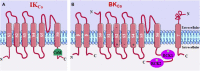
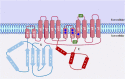
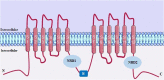
Asthma is one of the main non-communicable chronic diseases and affects a huge portion of the population. It is a multifactorial disease, classified into several phenotypes, being the allergic the most frequent. The pathophysiological mechanism of asthma involves a Th2-type immune response, with high concentrations of allergen-specific immunoglobulin E, eosinophilia, hyperreactivity and airway remodeling. These mechanisms are orchestrated by intracellular signaling from effector cells, such as lymphocytes and eosinophils. Ion channels play a fundamental role in maintaining the inflammatory response on asthma. In particular, transient receptor potential (TRP), stock-operated Ca2+ channels (SOCs), Ca2+-activated K+ channels (IKCa and BKCa), calcium-activated chloride channel (TMEM16A), cystic fibrosis transmembrane conductance regulator (CFTR), piezo-type mechanosensitive ion channel component 1 (PIEZO1) and purinergic P2X receptor (P2X). The recognition of the participation of these channels in the pathological process of asthma is important, as they become pharmacological targets for the discovery of new drugs and/or pharmacological tools that effectively help the pharmacotherapeutic follow-up of this disease, as well as the more specific mechanisms involved in worsening asthma.
Keywords: CFTR; KCa channels; ORAI channels; P2X receptor; Piezo1 channel; TMEM16A channel; TRP channels; asthma.
Copyright © 2023 Figueiredo, Ferreira, Fernandes, Silva, Vasconcelos and Cavalcante.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures




References
-
- Aleksandrov L., Aleksandrov A. A., Chang X. B., Riordan J. R. (2002). The first nucleotide binding domain of cystic fibrosis transmembrane conductance regulator is a site of stable nucleotide interaction, whereas the second is a site of rapid turnover. J. Biol. Chem. 277, 15419–15425. 10.1074/jbc.M111713200 - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

