Clickable Polyprolines from Azido-proline N-Carboxyanhydride
- PMID: 37841952
- PMCID: PMC10571246
- DOI: 10.1021/acspolymersau.3c00011
Clickable Polyprolines from Azido-proline N-Carboxyanhydride
Abstract
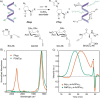
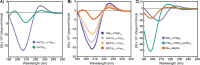
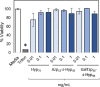
Polyproline is a material of great interest in biomedicine due to its helical scaffold of structural importance in collagen and mucins and its ability to gel and to change conformations in response to temperature. Appending of function-modulating chemical groups to such a material is desirable to diversify potential applications. Here, we describe the synthesis of high-molecular-weight homo, block, and statistical polymers of azide-functionalized proline. The azide groups served as moieties for highly efficient click-grafting, as stabilizers of the polyproline PPII helix, and as modulators of thermoresponsiveness. Saccharides and ethylene glycol were utilized to explore small-molecule grafting, and glutamate polymers were utilized to form polyelectrolyte bottlebrush architectures. Secondary structure effects of both the azide and click modifications, as well as lower critical solution temperature behavior, were characterized. The polyazidoprolines and click products were well tolerated by live human cells and are expected to find use in diverse biomedical applications.
© 2023 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures




References
-
- Traub W.; Shmueli U. Structure of Poly-L-Proline I. Nature 1963, 198, 1165–1166. 10.1038/1981165a0. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources
