Psychosocial stress-induced intestinal permeability in healthy humans: What is the evidence?
- PMID: 37842017
- PMCID: PMC10569989
- DOI: 10.1016/j.ynstr.2023.100579
Psychosocial stress-induced intestinal permeability in healthy humans: What is the evidence?
Abstract
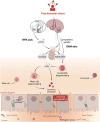
An impaired intestinal barrier function can be detrimental to the host as it may allow the translocation of luminal antigens and toxins into the subepithelial tissue and bloodstream. In turn, this may cause local and systemic immune responses and lead to the development of pathologies. In vitro and animal studies strongly suggest that psychosocial stress is one of the factors that can increase intestinal permeability via mast-cell dependent mechanisms. Remarkably, studies have not been able to yield unequivocal evidence that such relation between stress and intestinal permeability also exists in (healthy) humans. In the current Review, we discuss the mechanisms that are involved in stress-induced intestinal permeability changes and postulate factors that influence these alterations and that may explain the translational difficulties from in vitro and animal to human studies. As human research differs highly from animal research in the extent to which stress can be applied and intestinal permeability can be measured, it remains difficult to draw conclusions about the presence of a relation between stress and intestinal permeability in (healthy) humans. Future studies should bear in mind these difficulties, and more research into in vivo methods to assess intestinal permeability are warranted.
Keywords: CRH; Cortisol; Intestinal permeability; Mast cells; Psychosocial stress.
© 2023 The Authors. Published by Elsevier Inc.
Conflict of interest statement
Authors D.L.T., L.V.O., T.V., and K.V. declare none.
Figures

Similar articles
-
Psychological stress and corticotropin-releasing hormone increase intestinal permeability in humans by a mast cell-dependent mechanism.Gut. 2014 Aug;63(8):1293-9. doi: 10.1136/gutjnl-2013-305690. Epub 2013 Oct 23. Gut. 2014. PMID: 24153250
-
Chronological assessment of mast cell-mediated gut dysfunction and mucosal inflammation in a rat model of chronic psychosocial stress.Brain Behav Immun. 2010 Oct;24(7):1166-75. doi: 10.1016/j.bbi.2010.06.002. Epub 2010 Jun 25. Brain Behav Immun. 2010. PMID: 20600818
-
Corticotropin-releasing hormone (CRH) regulates macromolecular permeability via mast cells in normal human colonic biopsies in vitro.Gut. 2008 Jan;57(1):50-8. doi: 10.1136/gut.2006.117549. Epub 2007 May 24. Gut. 2008. PMID: 17525093
-
Review on the effect of chemotherapy on the intestinal barrier: Epithelial permeability, mucus and bacterial translocation.Biomed Pharmacother. 2023 Jun;162:114644. doi: 10.1016/j.biopha.2023.114644. Epub 2023 Apr 3. Biomed Pharmacother. 2023. PMID: 37018992 Review.
-
Development, validation and implementation of an in vitro model for the study of metabolic and immune function in normal and inflamed human colonic epithelium.Dan Med J. 2015 Jan;62(1):B4973. Dan Med J. 2015. PMID: 25557335 Review.
Cited by
-
Restraint Stress-Induced Neutrophil Inflammation Contributes to Concurrent Gastrointestinal Injury in Mice.Int J Mol Sci. 2024 May 11;25(10):5261. doi: 10.3390/ijms25105261. Int J Mol Sci. 2024. PMID: 38791301 Free PMC article.
-
Decreased Systemic Monocyte Colony Protein-1 (MCP-1) Levels and Reduced sCD14 Levels in Curcumin-Treated Patients with Moderate Anxiety: A Pilot Study.Antioxidants (Basel). 2024 Aug 29;13(9):1052. doi: 10.3390/antiox13091052. Antioxidants (Basel). 2024. PMID: 39334711 Free PMC article.
-
Dysregulated brain-gut axis in the setting of traumatic brain injury: review of mechanisms and anti-inflammatory pharmacotherapies.J Neuroinflammation. 2024 May 10;21(1):124. doi: 10.1186/s12974-024-03118-3. J Neuroinflammation. 2024. PMID: 38730498 Free PMC article. Review.
-
Endogenous Ethanol Production in the Human Alimentary Tract: A Literature Review.J Gastroenterol Hepatol. 2025 Apr;40(4):783-790. doi: 10.1111/jgh.16869. Epub 2025 Jan 23. J Gastroenterol Hepatol. 2025. PMID: 39853762 Free PMC article. Review.
-
Effects of psychosocial stress on laryngeal microbiology and epithelial barrier integrity.Sci Rep. 2025 Jul 12;15(1):25278. doi: 10.1038/s41598-025-10170-3. Sci Rep. 2025. PMID: 40652088 Free PMC article.
References
Publication types
LinkOut - more resources
Full Text Sources
Research Materials

