Hyperactive KRAS/MAPK signaling disrupts normal lymphatic vessel architecture and function
- PMID: 37842094
- PMCID: PMC10571159
- DOI: 10.3389/fcell.2023.1276333
Hyperactive KRAS/MAPK signaling disrupts normal lymphatic vessel architecture and function
Abstract
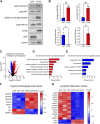
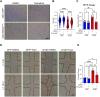
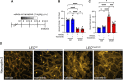
Complex lymphatic anomalies (CLAs) are sporadically occurring diseases caused by the maldevelopment of lymphatic vessels. We and others recently reported that somatic activating mutations in KRAS can cause CLAs. However, the mechanisms by which activating KRAS mutations cause CLAs are poorly understood. Here, we show that KRASG12D expression in lymphatic endothelial cells (LECs) during embryonic development impairs the formation of lymphovenous valves and causes the enlargement of lymphatic vessels. We demonstrate that KRASG12D expression in primary human LECs induces cell spindling, proliferation, and migration. It also increases AKT and ERK1/2 phosphorylation and decreases the expression of genes that regulate the maturation of lymphatic vessels. We show that MEK1/2 inhibition with the FDA-approved drug trametinib suppresses KRASG12D-induced morphological changes, proliferation, and migration. Trametinib also decreases ERK1/2 phosphorylation and increases the expression of genes that regulate the maturation of lymphatic vessels. We also show that trametinib and Cre-mediated expression of a dominant-negative form of MEK1 (Map2k1 K97M) suppresses KRASG12D-induced lymphatic vessel hyperplasia in embryos. Last, we demonstrate that conditional knockout of wild-type Kras in LECs does not affect the formation or function of lymphatic vessels. Together, our data indicate that KRAS/MAPK signaling must be tightly regulated during embryonic development for the proper development of lymphatic vessels and further support the testing of MEK1/2 inhibitors for treating CLAs.
Keywords: Gorham-Stout disease; KRAS; complex lymphatic anomaly; lymphangiogenesis; lymphatic malformation; trametinib.
Copyright © 2023 Fernandes, Tresemer, Zhang, Rios, Scallan and Dellinger.
Conflict of interest statement
MD is the Director of Research for the Lymphatic Malformation Institute. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures






References
-
- Cha B., Geng X., Mahamud M. R., Fu J., Mukherjee A., Kim Y., et al. (2016). Mechanotransduction activates canonical Wnt/β-catenin signaling to promote lymphatic vascular patterning and the development of lymphatic and lymphovenous valves. Genes. Dev. 30 (12), 1454–1469. 10.1101/gad.282400.116 - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

