Multifactorial assessment of Parkinson's disease course and outcomes using trajectory modeling in a multiethnic, multisite cohort - extension of the LONG-PD study
- PMID: 37842125
- PMCID: PMC10569724
- DOI: 10.3389/fnagi.2023.1240971
Multifactorial assessment of Parkinson's disease course and outcomes using trajectory modeling in a multiethnic, multisite cohort - extension of the LONG-PD study
Abstract
Background: The severity, progression, and outcomes of motor and non-motor symptoms in Parkinson's disease (PD) are quite variable. Following PD cohorts holds promise for identifying predictors of disease severity and progression.
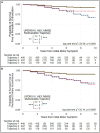
Methods: PD patients (N = 871) were enrolled at five sites. Enrollment occurred within 5 years of initial motor symptom onset. Disease progression was assessed annually for 2-to-10 years after onset. Group-based trajectory modeling was used to identify groups differing in disease progression. Models were developed for UPDRS-III scores, UPDRS-III tremor and bradykinesia-rigidity subscores, Hoehn & Yahr (H&Y) stage, Mini-Mental Status Exam (MMSE) scores, and UPDRS-III, H&Y and MMSE scores considered together. Predictors of trajectory-group membership were modeled simultaneously with the trajectories. Kaplan-Meier survival analysis evaluated survival free of PD outcomes.
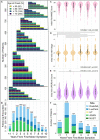
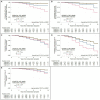
Results: The best fitting models identified three groups. One showed a relatively benign, slowly progressing trajectory (Group 1), a second showed a moderate, intermediately progressing trajectory (Group 2), and a third showed a more severe, rapidly progressing trajectory (Group 3). Stable trajectory-group membership occurred relatively early in the disease course, 5 years after initial motor symptom. Predictors of intermediate and more severe trajectory-group membership varied across the single variable models and the multivariable model jointly considering UPDRS-III, H&Y and MMSE scores. In the multivariable model, membership in Group 2 (28.4% of patients), relative to Group 1 (50.5%), was associated with male sex, younger age-at-onset, fewer education-years, pesticide exposure, absence of reported head injury, and akinetic/rigid subtype at initial presentation. Membership in Group 3 (21.3%), relative to Group 1, was associated with older age-at-onset, fewer education-years, pesticide exposure, and the absence of a tremor-predominant subtype at initial presentation. Persistent freezing, persistent falls, and cognitive impairment occurred earliest and more frequently in Group 3, later and less frequently in Group 2, and latest and least frequently in Group 1. Furthermore, autonomic complications, dysphagia, and psychosis occurred more frequently in Groups 2 and 3 than in Group 1.
Conclusion: Modeling disease course using multiple objective assessments over an extended follow-up duration identified groups that more accurately reflect differences in PD course, prognosis, and outcomes than assessing single parameters over shorter intervals.
Keywords: Parkinson’s disease; disease outcomes; group-based-trajectory model; longitudinal monitoring; motor symptoms; non-motor symptoms.
Copyright © 2023 Chase, Krueger, Pavelka, Chung, Aasly, Dardiotis, Premkumar, Schoneburg, Kartha, Aunaetitrakul, Frigerio, Maraganore and Markopoulou.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
-
- Bartl M., Dakna M., Schade S., Wicke T., Lang E., Ebentheuer J., et al. (2022). Longitudinal change and progression indicators using the movement disorder society-unified Parkinson's disease rating scale in two independent cohorts with early Parkinson's disease. J. Parkinsons Dis. 12, 437–452. doi: 10.3233/JPD-212860 - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

