Neutralising antibody responses against SARS-CoV-2 Omicron BA.4/5 and wild-type virus in patients with inflammatory bowel disease following three doses of COVID-19 vaccine (VIP): a prospective, multicentre, cohort study
- PMID: 37842172
- PMCID: PMC10570718
- DOI: 10.1016/j.eclinm.2023.102249
Neutralising antibody responses against SARS-CoV-2 Omicron BA.4/5 and wild-type virus in patients with inflammatory bowel disease following three doses of COVID-19 vaccine (VIP): a prospective, multicentre, cohort study
Abstract
Background: Patients with inflammatory bowel disease (IBD) receiving anti-TNF and JAK-inhibitor therapy have attenuated responses to COVID-19 vaccination. We aimed to determine how IBD treatments affect neutralising antibody responses against the Omicron BA.4/5 variant.
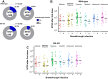
Methods: In this multicentre cohort study, we prospectively recruited 340 adults (69 healthy controls and 271 IBD) at nine UK hospitals between May 28, 2021 and March 29, 2022. The IBD study population was established (>12 weeks therapy) on either thiopurine (n = 63), infliximab (n = 45), thiopurine and infliximab combination therapy (n = 48), ustekinumab (n = 45), vedolizumab (n = 46) or tofacitinib (n = 24). Patients were excluded if they were being treated with any other immunosuppressive therapies. Participants had two doses of either ChAdOx1 nCoV-19 or BNT162b2 vaccines, followed by a third dose of either BNT162b2 or mRNA1273. Pseudo-neutralisation assays against SARS-CoV-2 wild-type and BA.4/5 were performed. The half maximal inhibitory concentration (NT50) of participant sera was calculated. The primary outcome was anti-SARS-CoV-2 neutralising response against wild-type virus and Omicron BA.4/5 variant after the second and third doses of anti-SARS-CoV-2 vaccine, stratified by immunosuppressive therapy, adjusting for prior infection, vaccine type, age, and interval between vaccination and blood collection. This study is registered with ISRCTN (No. 13495664).
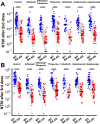
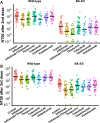
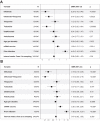
Findings: Both heterologous (first two doses adenovirus vaccine, third dose mRNA vaccine) and homologous (three doses mRNA vaccine) vaccination strategies significantly increased neutralising titres against both wild-type SARS-CoV-2 virus and the Omicron BA.4/5 variant in healthy participants and patients with IBD. Antibody titres against BA.4/5 were significantly lower than antibodies against wild-type virus in both healthy participants and patients with IBD (p < 0.0001). Multivariable models demonstrated that neutralising antibodies against BA.4/5 after three doses of vaccine were significantly lower in patients with IBD on infliximab (Geometric Mean Ratio (GMR) 0.19 [0.10, 0.36], p < 0.0001), infliximab and thiopurine combination (GMR 0.25 [0.13, 0.49], p < 0.0001) or tofacitinib (GMR 0.43 [0.20, 0.91], p = 0.028), but not in patients on thiopurine monotherapy, ustekinumab, or vedolizumab. Breakthrough infection was associated with lower neutralising antibodies against wild-type (p = 0.037) and BA.4/5 (p = 0.045).
Interpretation: A third dose of a COVID-19 mRNA vaccine based on the wild-type spike glycoprotein significantly boosts neutralising antibody titres in patients with IBD. However, responses are lower against the Omicron variant BA.4/5, particularly in patients taking anti-TNF and JAK-inhibitor therapy. Breakthrough infections are associated with lower neutralising antibodies and immunosuppressed patients with IBD may receive additional benefit from bivalent vaccine boosters which target Omicron variants.
Funding: Pfizer.
Keywords: Breakthrough infection; Inflammatory bowel disease; Infliximab; SARS-CoV-2; Tofacitinib.
© 2023 The Author(s).
Conflict of interest statement
ALH reports payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing, or educational events from AbbVie, Arena, Atlantic, Bristol Myers Squibb, Celltrion, Ferring, Dr Falk, Galapagos, Janssen, MSD, Napp, Pfizer, Pharmacosmos, Shire, and Takeda; participation on the Global Steering Committee for Genentech. AJK reports consulting fees from Janssen; payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing, or educational events from Pfizer and Takeda; support for attending meetings or travel from Janssen, Tillotts, and Norgine; and participation in a data safety monitoring board or advisory board for AbbVie. CWL received personal consulting fees from Galapagos, AbbVie, Takeda, Pfizer, Janssen and Iterative Scopes; institutional consulting fees from Trellus Health; and support for attending meetings from Galapagos, AbbVie, Takeda, Pfizer, Janssen, GSK, Gilead, Fresenius Kabi, Ferring, and Dr Falk. GRJ received speaker fees from Takeda, Ferring, Janssen, AbbVie and Fresenius Kabi. JLA received support for attending meetings from Takeda. JRG reports grants from Roche, Biogen, Celltrion Healthcare, Takeda and Galapagos. KVP received consulting fees from AbbVie, Janssen, Galapagos and Pfizer; reports payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing, or educational events from AbbVie, Dr Falk, Janssen, Galapagos, Pfizer, Takeda, Tillotts and Ferring; support for attending meetings or travel from AbbVie, Dr Falk, Janssen, Galapagos, Pfizer, Takeda, Tillotts and Ferring; and participation on a data safety monitoring board or advisory board for AbbVie, Galapagos, Pfizer and Janssen. SB received research grant from Bowel Research UK and support for attending conferences from Ferring and Dr Falk, outside the submitted work. KMP is a member of the data safety monitoring board for NCT05249829 and NCT05575492; has received a fee for speaking from Seqirus and Sanofi Pasteur, and has research funding from the Chan Zuckerberg Initiative, the MRC/UKRI, the Vaccine Task Force, and NIHR Imperial BRC outside the submitted work. KK reports payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing, or educational events from Janssen and Ferring; support for attending meetings or travel from Janssen and Takeda; and participation on a data safety monitoring board or advisory board for Janssen and PredictImmune. MP received research grant from Pfizer and speaker fees from Janssen. NAK reports grants from AbbVie, Biogen, Celgene, Celltrion, Galapagos, MSD, Napp, Pfizer, Pharmacosmos, Roche, and Takeda; consulting fees from Amgen, Bristol Myers Squibb, Dr Falk, Janssen, Mylan, Pharmacosmos, Galapagos, Takeda, and Tillotts; personal fees from Allergan, Celltrion, Dr Falk, Ferring, Janssen, Pharmacosmos, Takeda, Tillotts, and Galapagos; and support for attending meetings from AbbVie, Dr Falk, Tillotts and Janssen, outside the submitted work. PMI reports grants from Celltrion, Takeda, MSD, Pfizer, and Galapagos; personal fees from Gilead, Pfizer, Galapagos, Takeda, AbbVie, Celltrion, Janssen, Bristol Myers Squibb, Lilly, and Arena; speaker fees from Pfizer, Galapagos, Takeda, AbbVie, Celltrion, Janssen, Bristol Myers Squibb, Lilly, and Arena, outside the submitted work. SS reports grants from Tillotts, Takeda, Janssen, Pfizer and AbbVie; received personal fees from AbbVie, Tillotts, Janssen, Takeda, Dr Falk, Lilly and Bristol Myers Squibb; received support for attending meetings from Janssen, AbbVie and Celltrion; and serves as a member of the data safety monitoring board for AbbVie, Janssen, Takeda, Celltrion, Lilly and Bristol Myers Squibb. TA reports grant funding from Pfizer to deliver this study; grants from Celltrion, Roche, Takeda, Biogen, and Galapagos; and honoraria for lectures from Takeda and Roche, outside the submitted work. NP is the principal investigator on the research grant from Pfizer that funded the VIP study; has received research grants from Bristol Myers Squibb, Roche, Biogen, Celltrion Healthcare, Takeda, Galapagos, CCUK, AstraZeneca, Helmsley Charitable Trust, outside the submitted work; reports personal fees from Takeda, Janssen, Pfizer, Galapagos, Bristol Myers Squibb, AbbVie, Roche, Lilly, Allergan, Celgene and AstraZeneca outside the submitted work; and has served as a speaker or advisory board member for AbbVie, Allergan, Bristol Myers Squibb, Celgene, Dr Falk, AstraZeneca, Galapagos and Vifor; and serves as a member of the data safety monitoring board for Bristol Myers Squibb and AstraZeneca. All other authors declare no competing interests.
Figures


References
-
- Haas E.J., Angulo F.J., McLaughlin J.M., et al. Impact and effectiveness of mRNA BNT162b2 vaccine against SARS-CoV-2 infections and COVID-19 cases, hospitalisations, and deaths following a nationwide vaccination campaign in Israel: an observational study using national surveillance data. Lancet. 2021;397(10287):1819–1829. - PMC - PubMed
-
- Chemaitelly H., Yassine H.M., Benslimane F.M., et al. mRNA-1273 COVID-19 vaccine effectiveness against the B.1.1.7 and B.1.351 variants and severe COVID-19 disease in Qatar. Nat Med. 2021;27(9):1614–1621. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

