RIFLE: a Phase II trial of stereotactic ablative radiotherapy combined with fruquintinib and tislelizumab in metastatic colorectal cancer
- PMID: 37842200
- PMCID: PMC10568524
- DOI: 10.1093/gastro/goad063
RIFLE: a Phase II trial of stereotactic ablative radiotherapy combined with fruquintinib and tislelizumab in metastatic colorectal cancer
Abstract
Background: Currently, the prognosis for metastatic colorectal cancer (mCRC) still remains poor. The management of mCRC has become manifold because of the varied advances in the systemic and topical treatment approaches. For patients with limited number of metastases, radical local therapy plus systemic therapy can be a good choice to achieve long-term tumor control. In this study, we aimed to explore the efficacy and safety of the combination of fruquintinib, tislelizumab, and stereotactic ablative radiotherapy (SABR) in mCRC (RIFLE study).
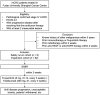
Methods: RIFLE was designed as a single-center, single-arm, prospective Phase II clinical trial. A total of 68 mCRC patients who have failed the first-line standard treatment will be recruited in the safety run-in phase (n = 6) and the expansion phase (n = 62), respectively. Eligible patients will receive SABR followed by fruquintinib (5 mg, d1-14, once every day) and tislelizumab (200 mg, d1, once every 3 weeks) within 2 weeks from completion of radiation. The expansion phase starts when the safety of the treatment is determined (dose limiting toxicity occur in no more than one-sixth of patients in the run-in phase). The primary end point is the objective response rate. The secondary end points include the disease control rate, duration of response, 3-year progression-free survival rate, 3-year overall survival rate, and toxicity.
Conclusions: The results of this trial will provide a novel insight into SABR in combination with PD-1 antibody and vascular endothelial growth factor receptor inhibitor in the systematic treatment of metastatic colorectal cancer, which is expected to provide new therapeutic strategies and improve the prognosis for mCRC patients.
Trial registration: NCT04948034 (ClinicalTrials.gov).
Keywords: SABR; immunotherapy; metastatic colorectal cancer.
© The Author(s) 2023. Published by Oxford University Press and Sixth Affiliated Hospital of Sun Yat-sen University.
Conflict of interest statement
Hutchison MediPharma Co., Ltd and BeiGene (Beijing) Co., Ltd provided drug and financial support for the study; they did not have any role in the design of the study and will not have any role in the collection, analysis, and interpretation of data or in writing the manuscript. The authors declare that they have no other conflict of interests in this study. Table 1.Tislelizumab administration adjustment schemeirAESeverityAdjustmentPneumoniaG2 pneumoniaHoldRecurrent G2 pneumonia, G3–4 pneumoniaPermanently discontinueDiarrhea/colitisG2–3 diarrhea/colitisHoldG4 diarrhea/colitisPermanently discontinueHepatitisG2 hepatitis, 3 ULN ≤ ALT/AST ≤ 5 ULN or 1.5 ULN ≤ TBIL ≤ 3 ULNHoldG3–4 hepatitis, ALT/AST ≥ 5 ULN or TBIL ≥ 3 ULNPermanently discontinueRenal insufficiencyG2–3 elevated serum creatinineHoldG4 elevated serum creatininePermanently discontinueEndocrine adverse eventsClinical G2–3 hypothyroidism/thyrotoxicosisG2–3 hypophysitisG2 adrenal insufficiencyG3 hyperglycemia or type I diabetes mellitusHoldG4 hypothyroidism/thyrotoxicosisG4 hypophysitisG4 adrenal insufficiencyG4 hyperglycemia or type I diabetes mellitusPermanently discontinueDermal toxicityG3 dermatitisHoldG4 dermatitis, Stevens–Johnson syndrome, or toxic epidermal necrolysisPermanently discontinueThrombocytopeniaG3 thrombocytopeniaHoldOther irAEsG3–4 elevation in amylase/lipaseG2–3 pancreatitisG2 myocarditisOther G2–3 irAEs occurred for the first timeHoldG4 pancreatitis or recurrent pancreatitis of any gradeG3–4 myocarditisG3–4 encephalitisOther G4 irAEs occurred for the first timePermanently discontinueRecurrent or persistent AERecurrent G3–4 AE (except for endocrine disease)G2–3 AEs not return to grade 0–1 or baseline levels within 12 weeks after the last dose (except for endocrine disease)Glucocorticoids not decrease to a prednisone equivalent dose of <10 mg/day within 12 weeks after the last dosePermanently discontinueInfusion-related reactionsG2 infusion-related reactionsSlow the rate of infusion or hold immunotherapyG3–4 infusion-related reactionsPermanently discontinueThe above adverse event severity evaluation is based on NCI-CTCAE v.5. irAE = immune-related adverse event, G1/2/3/4 = grade 1/2/3/4, ULN = upper limit of normal, ALT = alanine aminotransferase, AST = aspartate aminotransferase, TBIL = total bilirubin, AE = adverse event.
Figures
References
-
- Siegel RL, Miller KD, Fuchs HE et al. Cancer statistics, 2022. CA Cancer J Clin 2022;72:7–33. - PubMed
-
- Guckenberger M, Lievens Y, Bouma AB et al. Characterisation and classification of oligometastatic disease: a European Society for Radiotherapy and Oncology and European Organisation for Research and Treatment of Cancer consensus recommendation. Lancet Oncol 2020;21:e18–e28. - PubMed
Associated data
LinkOut - more resources
Full Text Sources
Medical

