Monitoring and responding to signals of suicidal ideation in pragmatic clinical trials: Lessons from the GRACE trial for Chronic Sickle Cell Disease Pain
- PMID: 37842321
- PMCID: PMC10569945
- DOI: 10.1016/j.conctc.2023.101218
Monitoring and responding to signals of suicidal ideation in pragmatic clinical trials: Lessons from the GRACE trial for Chronic Sickle Cell Disease Pain
Abstract
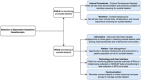
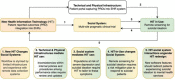
Sickle cell disease (SCD) is a hemoglobin disorder and the most common genetic disorder that affects 100,000 Americans and millions worldwide. Adults living with SCD have pain so severe that it often requires opioids to keep it in control. Depression is a major global public health concern associated with an increased risk in chronic medical disorders, including in adults living with sickle cell disease (SCD). A strong relationship exists between suicidal ideation, suicide attempts, and depression. Researchers enrolling adults living with SCD in pragmatic clinical trials are obligated to design their methods to deliberately monitor and respond to symptoms related to depression and suicidal ideation. This will offer increased protection for their participants and help clinical investigators meet their fiduciary duties. This article presents a review of this sociotechnical milieu that highlights, analyzes, and offers recommendations to address ethical considerations in the development of protocols, procedures, and monitoring activities related to suicidality in depressed patients in a pragmatic clinical trial.
Keywords: Depression; Patient-reported outcomes; Pragmatic trial; Research ethics; Suicidal ideation; Suicidal monitoring.
© 2023 The Authors.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures


Similar articles
-
Depression, suicidal ideation, and attempts in black patients with sickle cell disease.J Natl Med Assoc. 2009 Nov;101(11):1090-5. doi: 10.1016/s0027-9684(15)31103-2. J Natl Med Assoc. 2009. PMID: 19998636
-
Responding to signals of mental and behavioral health risk in pragmatic clinical trials: Ethical obligations in a healthcare ecosystem.Contemp Clin Trials. 2022 Feb;113:106651. doi: 10.1016/j.cct.2021.106651. Epub 2022 Jan 5. Contemp Clin Trials. 2022. PMID: 34998990 Free PMC article.
-
The controversial link between antidepressants and suicidality risks in adults: data from a naturalistic study on a large sample of in-patients with a major depressive episode.Int J Neuropsychopharmacol. 2009 Mar;12(2):181-9. doi: 10.1017/S1461145708009139. Epub 2008 Jul 29. Int J Neuropsychopharmacol. 2009. PMID: 18662490 Clinical Trial.
-
Clinical predictors of suicidal ideation, suicide attempts and suicide death in depressive disorder: a systematic review and meta-analysis.Eur Arch Psychiatry Clin Neurosci. 2024 Oct;274(7):1543-1563. doi: 10.1007/s00406-023-01716-5. Epub 2023 Nov 28. Eur Arch Psychiatry Clin Neurosci. 2024. PMID: 38015265 Free PMC article.
-
[Social pain at the core of suicidal behavior].Encephale. 2019 Jan;45 Suppl 1:S7-S12. doi: 10.1016/j.encep.2018.09.005. Epub 2018 Nov 11. Encephale. 2019. PMID: 30428996 Review. French.
Cited by
-
What interval of daily pain assessment is required to reliably diagnose chronic pain in SCD? The Pain in Sickle Cell Epidemiology Study.J Sick Cell Dis. 2024 Oct 23;1(1):yoae011. doi: 10.1093/jscdis/yoae011. eCollection 2024. J Sick Cell Dis. 2024. PMID: 40304011 Free PMC article.
References
-
- Data & Statistics on Sickle Cell Disease. CDC, Centers for Disease Control and Prevention; 2022. https://www.cdc.gov/ncbddd/sicklecell/data.html
-
- Major depression, national Institute of mental health (NIMH) https://www.nimh.nih.gov/health/statistics/major-depression n.d.
Grants and funding
LinkOut - more resources
Full Text Sources

