Topical Spironolactone in the Treatment of Ocular Graft-Versus-Host Disease
- PMID: 37842497
- PMCID: PMC10569898
- DOI: 10.7759/cureus.45136
Topical Spironolactone in the Treatment of Ocular Graft-Versus-Host Disease
Abstract
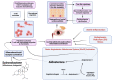
Introduction: This two-part study aimed to investigate the therapeutic potential of topical spironolactone in ocular graft-versus-host disease (oGVHD). While off-label use of topical spironolactone has been described in dry eye, its efficacy in managing signs and symptoms of oGVHD remains unstudied. Preclinically, we tested the hypothesis that spironolactone induces corneal lipid synthesis in a mouse model. Clinically, we assessed patient response to spironolactone with a retrospective observational design.
Methods: Both immortalized and primary human corneal epithelial cells were stained with oil red O after 9 days of treatment with spironolactone. C57BL/6 mice were dosed thrice daily with one drop in each eye for 18 days. Corneal tissue was stained with oil red O and BODIPY™. Twenty eyes with oGVHD, as defined by the International Chronic oGVHD Consensus Group, were studied. Corneal fluorescein staining, lid margin vascularity, meibomian gland obstruction, meibum turbidity, zone A posterior lid margin vascularity, and oGVHD diagnostic criteria severity grading were compared in a pre-post study. Follow-up times ranged from 7 to 21 weeks, with a median time of 12 weeks. Statistical analysis was done with STATA 17 by fitting data to a non-parametric model.
Results: In vitro results showed an increased number and density of oil red O staining granules in the treatment group versus control in both primary and immortalized human corneal epithelium. In vivo, results showed translation to the mouse model with increased corneal epithelial BODIPY™ signal compared to untreated control. oGVHD patients had improved lid margin vascularity (p = 0.046), corneal fluorescein staining (p = 0.021), and International oGVHD Consensus Group severity scores (p = 0.011) after treatment with topical spironolactone. Minimal adverse effects were noted, the most common being mild stinging lasting less than a minute after instillation.
Conclusion: The improved severity scores, lid margin inflammation, and corneal fluorescein staining after weeks of treatment support the rationale that topical spironolactone may benefit oGVHD. The observed lipid production by the corneal epithelium is thought to contribute to this protective effect against ocular surface erosive disease in oGVHD. A mineralocorticoid receptor antagonist, spironolactone may offer therapeutic benefits in oGVHD while avoiding undesirable side effects of topical or systemic glucocorticoids.
Keywords: cornea; dry eye disorder; ocular graft-versus-host disease; ocular keratitis; tear film.
Copyright © 2023, Wong et al.
Conflict of interest statement
We would like to disclose that RW Yee holds a patent for topical use of spironolactone. (US Patent Publication Number 20190328753).
Figures




Similar articles
-
Effects and Safety of 5% Lifitegrast Ophthalmic Solution in Patients With Dry Eye Disease Associated With Ocular Graft-Versus-Host Disease.Cureus. 2024 Aug 8;16(8):e66437. doi: 10.7759/cureus.66437. eCollection 2024 Aug. Cureus. 2024. PMID: 39246931 Free PMC article.
-
Application of lacrimal gland ultrasonography in the evaluation of chronic ocular graft-versus-host-disease.Front Immunol. 2025 Feb 5;16:1490390. doi: 10.3389/fimmu.2025.1490390. eCollection 2025. Front Immunol. 2025. PMID: 39975559 Free PMC article.
-
Efficacy and retention of silicone punctal plugs for treatment of dry eye in patients with and without ocular graft-versus-host-disease.Ocul Surf. 2020 Oct;18(4):731-735. doi: 10.1016/j.jtos.2020.07.018. Epub 2020 Jul 30. Ocul Surf. 2020. PMID: 32738300 Free PMC article.
-
Update on ocular graft-versus-host disease.Indian J Ophthalmol. 2021 May;69(5):1038-1050. doi: 10.4103/ijo.IJO_2016_20. Indian J Ophthalmol. 2021. PMID: 33913829 Free PMC article. Review.
-
Lessons Learned From Ocular Graft versus Host Disease: An Ocular Surface Inflammatory Disease of Known Time of Onset.Eye Contact Lens. 2024 May 1;50(5):212-221. doi: 10.1097/ICL.0000000000001082. Epub 2024 Mar 22. Eye Contact Lens. 2024. PMID: 38518064 Review.
Cited by
-
Advances in the ocular complications after hematopoietic stem cell transplantation.Ann Hematol. 2024 Oct;103(10):3867-3880. doi: 10.1007/s00277-024-05678-z. Epub 2024 Feb 26. Ann Hematol. 2024. PMID: 38403713 Review.
-
Topical Spironolactone in the Treatment of Evaporative Dry Eye Disease.Cureus. 2023 Jun 27;15(6):e41038. doi: 10.7759/cureus.41038. eCollection 2023 Jun. Cureus. 2023. PMID: 37519614 Free PMC article.
-
Therapeutic Targets in the Management of Dry Eye Disease Associated with Sjögren's Syndrome: An Updated Review of Current Insights and Future Perspectives.J Clin Med. 2024 Mar 20;13(6):1777. doi: 10.3390/jcm13061777. J Clin Med. 2024. PMID: 38542002 Free PMC article. Review.
-
Effects and Safety of 5% Lifitegrast Ophthalmic Solution in Patients With Dry Eye Disease Associated With Ocular Graft-Versus-Host Disease.Cureus. 2024 Aug 8;16(8):e66437. doi: 10.7759/cureus.66437. eCollection 2024 Aug. Cureus. 2024. PMID: 39246931 Free PMC article.
References
-
- Chronic graft-versus-host disease. Atkinson K. https://pubmed.ncbi.nlm.nih.gov/2178709/ Bone Marrow Transplant. 1990;5:69–82. - PubMed
-
- Evidence that large granular lymphocytes of donor origin mediate acute graft-versus-host disease. Ferrara JL, Guillen FJ, van Dijken PJ, Marion A, Murphy GF, Burakoff SJ. Transplantation. 1989;47:50–54. - PubMed
-
- Ocular manifestations of graft-vs-host disease. Franklin RM, Kenyon KR, Tutschka PJ, Saral R, Green WR, Santos GW. Ophthalmology. 1983;90:4–13. - PubMed
-
- Scleroderma, Sjögren-like syndrome, and chronic graft-versus-host disease. Lawley TJ, Peck GL, Moutsopoulos HM, Gratwohl AA, Deisseroth AB. Ann Intern Med. 1977;87:707–709. - PubMed
-
- Sjögren-type syndrome after allogeneic bone-marrow transplantation. Gratwohl AA, Moutsopoulos HM, Chused TM, Akizuki M, Wolf RO, Sweet JB, Deisseroth AB. https://www.acpjournals.org/doi/abs/10.7326/0003-4819-87-6-703. Ann Intern Med. 1977;87:703–706. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
