VIDIIA Hunter diagnostic platform: a low-cost, smartphone connected, artificial intelligence-assisted COVID-19 rapid diagnostics approved for medical use in the UK
- PMID: 37842636
- PMCID: PMC10572354
- DOI: 10.3389/fmolb.2023.1144001
VIDIIA Hunter diagnostic platform: a low-cost, smartphone connected, artificial intelligence-assisted COVID-19 rapid diagnostics approved for medical use in the UK
Erratum in
-
Corrigendum: VIDIIA Hunter: a low-cost, smartphone connected, artificial intelligence-assisted COVID-19 rapid diagnostic platform approved for medical use in the UK.Front Mol Biosci. 2023 Oct 31;10:1325104. doi: 10.3389/fmolb.2023.1325104. eCollection 2023. Front Mol Biosci. 2023. PMID: 38028554 Free PMC article.
Abstract
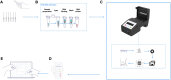
Introduction: Accurate and rapid diagnostics paired with effective tracking and tracing systems are key to halting the spread of infectious diseases, limiting the emergence of new variants and to monitor vaccine efficacy. The current gold standard test (RT-qPCR) for COVID-19 is highly accurate and sensitive, but is time-consuming, and requires expensive specialised, lab-based equipment. Methods: Herein, we report on the development of a SARS-CoV-2 (COVID-19) rapid and inexpensive diagnostic platform that relies on a reverse-transcription loop-mediated isothermal amplification (RT-LAMP) assay and a portable smart diagnostic device. Automated image acquisition and an Artificial Intelligence (AI) deep learning model embedded in the Virus Hunter 6 (VH6) device allow to remove any subjectivity in the interpretation of results. The VH6 device is also linked to a smartphone companion application that registers patients for swab collection and manages the entire process, thus ensuring tests are traced and data securely stored. Results: Our designed AI-implemented diagnostic platform recognises the nucleocapsid protein gene of SARS-CoV-2 with high analytical sensitivity and specificity. A total of 752 NHS patient samples, 367 confirmed positives for coronavirus disease (COVID-19) and 385 negatives, were used for the development and validation of the test and the AI-assisted platform. The smart diagnostic platform was then used to test 150 positive clinical samples covering a dynamic range of clinically meaningful viral loads and 250 negative samples. When compared to RT-qPCR, our AI-assisted diagnostics platform was shown to be reliable, highly specific (100%) and sensitive (98-100% depending on viral load) with a limit of detection of 1.4 copies of RNA per µL in 30 min. Using this data, our CE-IVD and MHRA approved test and associated diagnostic platform has been approved for medical use in the United Kingdom under the UK Health Security Agency's Medical Devices (Coronavirus Test Device Approvals, CTDA) Regulations 2022. Laboratory and in-silico data presented here also indicates that the VIDIIA diagnostic platform is able to detect the main variants of concern in the United Kingdom (September 2023). Discussion: This system could provide an efficient, time and cost-effective platform to diagnose SARS-CoV-2 and other infectious diseases in resource-limited settings.
Keywords: COVID-19; LAMP (loop mediated isothermal amplification); artificial intelligence; infectious diseases; rapid diagnostics.
Copyright © 2023 Poirier, Riaño Moreno, Takaindisa, Carpenter, Mehat, Haddon, Rohaim, Williams, Burkhart, Conlon, Wilson, McClumpha, Stedman, Cordoni, Branavan, Tharmakulasingam, Chaudhry, Locker, Fernando, Balachandran, Bullen, Collins, Rimer, Horton, Munir and La Ragione.
Conflict of interest statement
Authors RRM, JC, and DR were employed by the company VIDIIA Ltd. Authors CC, MW, MaM, and MBu were employed by the company GB Electronics (UK) Ltd. VIDIIA Ltd is start-up company formed in 2020, in which the University of Surrey owns shares and has an Investor Director in place on the company Board of Directors. AP and RL are scientific advisors for VIDIIA. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



References
-
- Alves P. A., de Oliveira E. G., Franco-Luiz A. P. M., Almeida L. T., Goncalves A. B., Borges I. A., et al. (2021). Optimization and clinical validation of colorimetric reverse transcription loop-mediated isothermal amplification, a fast, highly sensitive and specific COVID-19 molecular diagnostic tool that is robust to detect SARS-CoV-2 variants of concern. Front. Microbiol. 12, 713713. 10.3389/fmicb.2021.713713 - DOI - PMC - PubMed
-
- Baek Y. H., Um J., Antigua K. J. C., Park J. H., Kim Y., Oh S., et al. (2020). Development of a reverse transcription-loop-mediated isothermal amplification as a rapid early-detection method for novel SARS-CoV-2. Emerg. Microbes Infect. 9 (1), 998–1007. 10.1080/22221751.2020.1756698 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

