Exploring the role of urinary extracellular vesicles in kidney physiology, aging, and disease progression
- PMID: 37842748
- PMCID: PMC10861146
- DOI: 10.1152/ajpcell.00349.2023
Exploring the role of urinary extracellular vesicles in kidney physiology, aging, and disease progression
Abstract
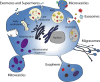
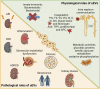
Extracellular vesicles (EVs), membranous vesicles present in all body fluids, are considered important messengers, carrying their information over long distance and modulating the gene expression profile of recipient cells. EVs collected in urine (uEVs) are mainly originated from the apical part of urogenital tract, following the urine flow. Moreover, bacterial-derived EVs are present within urine and may reflect the composition of microbiota. Consolidated evidence has established the involvement of uEVs in renal physiology, being responsible for glomerular and tubular cross talk and among different tubular segments. uEVs may also be involved in other physiological functions such as modulation of innate immunity, coagulation, or metabolic activities. Furthermore, it has been recently remonstrated that age, sex, endurance excise, and lifestyle may influence uEV composition and release, modifying their cargo. On the other hand, uEVs appear modulators of different urogenital pathological conditions, triggering disease progression. uEVs sustain fibrosis and inflammation processes, both involved in acute and chronic kidney diseases, aging, and stone formation. The molecular signature of uEVs collected from diseased patients can be of interest for understanding kidney physiopathology and for identifying diagnostic and prognostic biomarkers.
Keywords: EV release; exosomes; renal injury; renal tubular cells; urine.
Conflict of interest statement
C.G. and B.B. are members of the Task Force on Urinary Extracellular Vesicles of the ISEV community. None of the other authors has any conflicts of interest, financial or otherwise, to disclose.
Figures


References
-
- Valcz G, Újvári B, Buzás EI, Krenács T, Spisák S, Kittel Á, Tulassay Z, Igaz P, Takács I, Molnár B. Small extracellular vesicle DNA-mediated horizontal gene transfer as a driving force for tumor evolution: facts and riddles. Front Oncol 12: 945376, 2022. doi: 10.3389/fonc.2022.945376. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

