Immunomodulatory interventions for focal epilepsy
- PMID: 37842826
- PMCID: PMC10577807
- DOI: 10.1002/14651858.CD009945.pub3
Immunomodulatory interventions for focal epilepsy
Abstract
Background: This is an updated version of an original Cochrane Review published in 2013 (Walker 2013). Epilepsy is a common neurological disorder affecting 0.5% to 1% of the population. Pharmacological treatment remains the first choice to control epilepsy. However, up to 30% of people do not respond to drug treatment, and therefore do not achieve seizure remission. Experimental and clinical evidence supports a role for inflammatory pathway activation in the pathogenesis of epilepsy which, if effectively targeted by immunomodulatory interventions, highlights a potentially novel therapeutic strategy.
Objectives: To assess the efficacy and tolerability of immunomodulatory interventions on seizures, adverse effect profile, cognition, and quality of life, compared to placebo controls, when used as additional therapy for focal epilepsy in children and adults.
Search methods: For the latest update, we searched the following databases on 11 November 2021: Cochrane Register of Studies (CRS Web) and Medline (Ovid) 1946 to 10 November 2021. CRS Web includes randomised or quasi-randomised, controlled trials from PubMed, EMBASE, ClinicalTrials.gov, the World Health Organization International Clinical Trials Registry Platform (ICTRP), the Cochrane Central Register of Controlled Trials (CENTRAL), and the Specialized Registers of Cochrane Review Groups including Epilepsy. We placed no language restrictions. We reviewed the bibliographies of retrieved studies to search for additional reports of relevant studies.
Selection criteria: Randomised placebo-controlled trials of add-on immunomodulatory drug interventions, in which an adequate method of concealment of randomisation was used. The studies were double-, single- or unblinded. Eligible participants were children (aged over 2 years) and adults with focal epilepsy.
Data collection and analysis: We used standard methodological procedures expected by the Cochrane Collaboration. We assessed the following outcomes. 1. 50% or greater reduction in seizure frequency. 2. Seizure freedom. 3. Treatment withdrawal for any reason. 4. Quality of life. 5.
Adverse effects: We used an intention-to-treat (ITT) population for all primary analyses, and we presented results as risk ratios (RRs) with 95% confidence intervals (95% Cl).
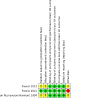
Main results: We included three randomised, double-blind, placebo-controlled trials on a total of 172 participants. All trials included children and adults over two years of age with focal epilepsy. Treatment phases lasted six weeks and follow-up from six weeks to six months. One of the three included trials described an adequate method of concealment of randomisation, whilst the other two trials were rated as having an unclear risk of bias due to lack of reported information around study design. Effective blinding of studies was reported in all three trials. All analyses were by ITT. One trial was sponsored by the manufacturer of an immunomodulatory agent and therefore was at high risk of funding bias. Immunomodulatory interventions were significantly more effective than placebo in reducing seizure frequency (risk ratio (RR) 2.30, 95% confidence interval (CI) 1.15 to 4.60; 3 studies, 172 participants; moderate-certainty evidence). For treatment withdrawal, there was insufficient evidence to conclude that people were more likely to discontinue immunomodulatory intervention than placebo (RR 1.04, 95% CI 0.28 to 3.80; 3 studies, 172 participants; low-certainty evidence). The RR for adverse effects was 1.16 (95% CI 0.84 to 1.59; 1 study, 66 participants; low-certainty evidence). Certain adverse effects such as dizziness, headache, fatigue, and gastrointestinal disorders were more often associated with immunomodulatory interventions. There were little to no data on cognitive effects and quality of life. No important heterogeneity between studies was found for any of the outcomes. We judged the overall certainty of evidence (using the GRADE approach) as low to moderate due to potential attrition bias resulting from missing outcome data and imprecise results with wide confidence intervals.
Authors' conclusions: Immunomodulatory interventions as add-on treatment for children and adults with focal epilepsy appear to be effective in reducing seizure frequency. It is not possible to draw any conclusions about the tolerability of these agents in children and adults with epilepsy. Further randomised controlled trials are needed.
Trial registration: ClinicalTrials.gov NCT01048255.
Copyright © 2023 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Conflict of interest statement
MP: none known.
LW: none known.
AGM: a consortium of pharmaceutical companies (GSK, EISAI, UCB Pharma) funded the National Audit of Seizure Management in Hospitals (NASH) through grants paid to the University of Liverpool. Professor Marson is funded in part by the NIHR Applied Research Collaboration, North West Coast (NIHR ARC NWC). Professor Marson is a National Institute for Health and Care Research (NIHR) Senior Investigator. The views expressed in this article are those of the author(s) and not necessarily those of the NIHR, or the Department of Health and Social Care. Professor Marson is the Co‐ordinating Editor of the Cochrane Epilepsy Group; however, he was not involved in the editorial process of this review update.
Figures







Update of
-
Immunomodulatory interventions for focal epilepsy syndromes.Cochrane Database Syst Rev. 2013 Jun 27;(6):CD009945. doi: 10.1002/14651858.CD009945.pub2. Cochrane Database Syst Rev. 2013. Update in: Cochrane Database Syst Rev. 2023 Oct 16;10:CD009945. doi: 10.1002/14651858.CD009945.pub3. PMID: 23803963 Updated.
References
References to studies included in this review
French 2012 {published and unpublished data}
-
- French J, Chen Y, Fan X, Hoock T, Martin M, Paskavitz J, et al. VX-765, a novel, investigational anti-inflammatory agent which inhibits IL-1beta production: proof-of-concept trial for refractory partial onset seizures. Epilepsy Currents 2012;12(Suppl 1):275.
French 2021 {published data only}
Van Rijckevorsel‐Harmant 1994 {published data only}
-
- Van Rijckevorsel-Harmant K, Delire M, Schmitz-Moorman W, Wieser HG. Treatment of refractory epilepsy with intravenous immunoglobulins. Results of the first double-blind/dose finding clinical study. International Journal of Clinical and Laboratory Research 1994;24(3):162-6. [PMID: ] - PubMed
References to studies excluded from this review
Barbaro 2009 {published data only}
-
- Barbaro NM, Quigg M, Broshek DK, Ward MM, Lamborn KR, Laxer KD, et al. A multicenter, prospective pilot study of gamma knife radiosurgery for mesial temporal lobe epilepsy: seizure response, adverse events, and verbal memory. Annals of Neurology 2009;65(2):167-75. [PMID: ] - PubMed
Barile‐Fabris 2005 {published data only}
-
- Barile-Fabris L, Ariza-Andraca R, Olguin-Ortega L, Jara LJ, Fraga-Mouret A, Miranda Limon JM, et al. Controlled clinical trial of IV cyclophosphamide versus IV methylprednisolone in severe neurological manifestations in systemic lupus erythematosus. Annals of the Rheumatic Diseases 2005;64(4):620-5. [PMID: ] - PMC - PubMed
Klein 1970 {published data only}
-
- Klein R. The effects of ACTH and corticosteroids on epileptiform disorders. Progress in Brain Research 1970;32:263-9. [PMID: ] - PubMed
Laxer 2000 {published data only}
-
- Laxer K, Blum D, Abou-Khalil BW, Morrell MJ, Lee DA, Data JL, et al. Assessment of ganaxolone's anticonvulsant activity using a randomized, double-blind, presurgical trial design. Ganaxolone Presurgical Study Group. Epilepsia 2000;41(9):1187-94. [PMID: ] - PubMed
Patel 2021 {published data only}
-
- Patel J, Feng W, Chen K, French JA, Rushton M, Hubbard S, et al. Use of an electronic seizure diary in a randomized, controlled trial of natalizumab in adult participants with drug-resistant focal epilepsy. Epilepsy and Behavior 2021;118:107925. [PMID: ] - PubMed
Zhao 2021 {published data only}
-
- Zhao Y, Epling D, Crass R, Rizzo M, Parkerson K, Chapel S, et al. Exposure-response (ER) analysis of natalizumab in subjects with drug-resistant focal epilepsy. Clinical Pharmacology and Therapeutics 2021;109(Suppl 1):S38. [DOI: 10.1002/cpt.2167] - DOI
References to studies awaiting assessment
Simko 1997 {published data only}
-
- Simko M, Mokrán V, Nyulassy S. Immunomodulatory therapy of epilepsy with transfer factor [Imunomodulacná terapia epilepsie transferovým faktorom]. Bratislavské lekárske listy 1997;98(4):234-7. [PMID: ] - PubMed
References to ongoing studies
CTRI/2021/02/030974 {published data only}
-
- CTRI/2021/02/030974. Efficacy and safety of prednisolone as an adjunct to antiepileptic drugs in drug resistant epilepsy: a randomized double blind placebo control study. www.ctri.nic.in/Clinicaltrials/pmaindet2.php?trialid=46961 (first received 5 February 2021).
NCT01545518 {unpublished data only}
-
- NCT01545518. IVIG treatment for refractory immune-related adult epilepsy. clinicaltrials.gov/ct2/show/NCT01545518 (first received 6 March 2012).
Additional references
Auvin 2010
Boer 2008
-
- Boer K, Jansen F, Nellist M, Redeker S, den Ouweland AM, Spliet WG, et al. Inflammatory processes in cortical tubers, and subependymal giant cell tumors of tuberous sclerosis complex. Epilepsy Research 2008;78(1):7–21. [PMID: ] - PubMed
Crespel 2002
-
- Crespel A, Coubes P, Rousset MC, Brana C, Rougier A, Rondouin G, et al. Inflammatory reactions in human medial temporal lobe epilepsy with hippocampal sclerosis. Brain Research 2002;952(2):159–69. [PMID: ] - PubMed
De Simoni 2000
-
- De Simoni MG, Perego C, Ravizza T, Moneta D, Conti M, Marchesi F, et al. Inflammatory cytokines and related genes are induced in the rat hippocampus by limbic status epilepticus. European Journal of Neuroscience 2000;12(7):2623–33. [PMID: ] - PubMed
Ehrchen 2019
EMEA 1995
-
- European Medicines Agency. ICH Topic E 2 A. Clinical Safety Data Management: Definitions and Standards for Expedited Reporting, 1995. www.emea.europa.eu/docs/en_GB/document_library/Scientific_guideline/2009... (accessed 1 May 2012).
Falco‐Walter 2018
-
- Falco-Walter JJ, Scheffer IE, Fisher RS. The new definition and classification of seizures and epilepsy. Epilepsy Res 2018;139:73-79. - PubMed
GRADEpro GDT [Computer program]
-
- GRADEpro GDT. Version accessed 9 May 2019. Hamilton (ON): McMaster University (developed by Evidence Prime), 2019. Available at gradepro.org.
Gupta 2017
Higgins 2011
-
- Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from training.cochrane.org/handbook/archive/v5.1/.
Hulkkonnen 2004
-
- Hulkkonnen J, Koskikallio E, Rainesalo S, Keranen T, Hurme M, Peltola J. The balance of inhibitory and excitatory cytokines is differently regulated in vivo and in vitro among therapy resistant epilepsy patients. Epilepsy Research 2004;59(2-3):199-205. [PMID: ] - PubMed
Kirkham 2010
Kwan 2000
-
- Kwan P, Brodie MJ. Early identification of refractory epilepsy. New England Journal of Medicine 2000;342(5):314-9. [PMID: ] - PubMed
Lefebvre 2021
-
- Lefebvre C, Glanville J, Briscoe S, Littlewood A, Marshall C, Metzendorf M-I, et al. Chapter 4: Searching for and selecting studies. In: Higgins JP, Thomas J, Chandler J, Cumpston MS, Li T, Page MJ, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.2 (updated February 2021). Cochrane, 2021. Available from www.training.cochrane.org/handbook.
Lehtimaki 2004
-
- Lehtimaki KA, Keranen T, Huhtala H, Hurme M, Ollikainen J, Honkaniemi J, et al. Regulation of IL-6 system in cerebrospinal fluid and serum compartments by seizures: the effect of seizure type and duration. Journal of Neuroimmunology 2004;152(1-2):121-5. [PMID: ] - PubMed
Marchi 2011
Panebianco 2015
Panebianco 2022
Peltola 1998
-
- Peltola J, Hurme M, Miettinen A, Keranen T. Elevated levels of interleukin-6 may occur in cerebrospinal fluid from patients with recent epileptic seizures. Epilepsy Research 1998;31(2):129-33. [PMID: ] - PubMed
Ravizza 2006
-
- Ravizza T, Boer K, Redeker S, Spliet WG, Rijen PC, Troost D, et al. The IL-1beta system in epilepsy-associated malformations of cortical development. Neurobiology of Disease 2006;24(1):128-43. [PMID: ] - PubMed
Ravizza 2008
-
- Ravizza T, Gagliardi B, Noe F, Boer K, Aronica E, Vezzani A. Innate and adaptive immunity during epileptogenesis and spontaneous seizures: evidence from experimental models and human temporal lobe epilepsy. Neurobiology of Disease 2008;29(1):142-60. [PMID: ] - PubMed
Review Manager 2020 [Computer program]
-
- Review Manager 5 (RevMan 5). Version 5.4. Copenhagen: The Cochrane Collaboration, 2020.
Scheffer 2017
Schűnemann 2013
-
- Schünemann H, Brożek J, Guyatt G, Oxman A, editor(s). Handbook for grading the quality of evidence and the strength of recommendations using the GRADE approach (updated October 2013). GRADE Working Group, 2013. Available from gdt.guidelinedevelopment.org/app/handbook/handbook.html.
Trinka 2023
Vezzani 2000
-
- Vezzani A, Moneta D, Conti M, Richichi C, Ravizza T, De Luigi A, et al. Powerful anticonvulsant action of IL-1 receptor antagonist on intracerebral injection and astrocytic overexpression in mice. Proceedings of the National Academy of Sciences of the United States of America 2000;97(21):11534-9. [PMID: ] - PMC - PubMed
Vezzani 2005a
Vezzani 2005b
-
- Vezzani A, Granata T. Brain inflammation in epilepsy: experimental and clinical evidence. Epilepsia 2005;46(11):1724-43. [PMID: ] - PubMed
Vezzani 2008
-
- Vezzani A, Balosso S, Ravizza T. The role of cytokines in the pathophysiology of epilepsy. Brain, Behavior, and Immunity 2008;22(6):797–803. [PMID: ] - PubMed
References to other published versions of this review
Walker 2012
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical

