Electroclinical features and phenotypic differences in adenylosuccinate lyase deficiency: Long-term follow-up of seven patients from four families and appraisal of the literature
- PMID: 37842880
- PMCID: PMC10839293
- DOI: 10.1002/epi4.12837
Electroclinical features and phenotypic differences in adenylosuccinate lyase deficiency: Long-term follow-up of seven patients from four families and appraisal of the literature
Abstract
Objective: Adenylosuccinate lyase (ADSL) deficiency is a rare inherited metabolic disorder with a wide phenotypic presentation, classically grouped into three types (neonatal, type I, and type II). We aim to better delineate the pathological spectrum, focusing on the electroclinical characteristics and phenotypic differences of patients with ADSL deficiency.
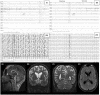
Patients and methods: Seven patients, from four different families, underwent serial electroencephalogram (EEG), clinical assessment, and neuroimaging. We also performed a systematic review of the cases published in the literature, summarizing the available clinical, neurophysiological, and genetic data.
Results: We report seven previously unreported ADSL deficiency patients with long-term follow-up (10-34 years). From the literature review, we collected 81 previously reported cases. Of the included patient population, 58 % (51/88) were classified as having ADSL deficiency type I, 28% (25/88) as having type II, and 14% (12/88) as having neonatal. The most frequently reported pathogenic variants are p.R426H homozygous (19 patients), p.Y114H in compound heterozygosity (13 patients), and p.D430N homozygous (6 patients). In the majority (89.2%), disease onset was within the first year of life. Epilepsy is present in 81.8% of the patients, with polymorphic and often intractable seizures. EEG features seem to display common patterns and developmental trajectories: (i) poor general background organization with theta-delta activity; (ii) hypsarrhythmia with spasms, usually adrenocorticotropic hormone-responsive; (iii) generalized epileptic discharges with frontal or frontal temporal predominance; and (iv) epileptic discharge activation in sleep with an altered sleep structure. Imaging features present consistent findings of cerebral atrophy with frontal predominance, cerebellar atrophy, and white matter abnormalities among the three types.
Significance: ADSL deficiency presents variable phenotypic expression, whose severity could be partially attributed to residual activity of the mutant protein. Although a precise phenotype-genotype correlation was not yet feasible, we delineated a common pattern of clinical, neuroradiological, and neurophysiological features.
Keywords: ADSL deficiency; EEG patterns; epilepsy; genotype-phenotype correlation; monogenic diseases.
© 2023 The Authors. Epilepsia Open published by Wiley Periodicals LLC on behalf of International League Against Epilepsy.
Conflict of interest statement
The authors have no disclosures related to the present work.
Figures


References
-
- Jaeken J, Van den Berghe G. An infantile autistic syndrome characterised by the presence of succinylpurines in body fluids. Lancet. 1984;324:1058–1061. - PubMed
-
- Jurecka A, Zikanova M, Jurkiewicz E, Tylki‐Szymańska A. Attenuated adenylosuccinate lyase deficiency: a report of one case and a review of the literature. Neuropediatrics. 2014;45:50–55. - PubMed
-
- Mouchegh K, Zikánová M, Hoffmann GF, Kretzschmar B, Kühn T, Mildenberger E, et al. Lethal fetal and early neonatal presentation of adenylosuccinate lyase deficiency: observation of 6 patients in 4 families. J Pediatr. 2007;150:57–61.e2. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

