Effect of efgartigimod on muscle group subdomains in participants with generalized myasthenia gravis: post hoc analyses of the phase 3 pivotal ADAPT study
- PMID: 37843174
- PMCID: PMC11235734
- DOI: 10.1111/ene.16098
Effect of efgartigimod on muscle group subdomains in participants with generalized myasthenia gravis: post hoc analyses of the phase 3 pivotal ADAPT study
Abstract
Background and purpose: Generalized myasthenia gravis (gMG) is a rare, chronic, neuromuscular autoimmune disease mediated by pathogenic immunoglobulin G (IgG) autoantibodies. Patients with gMG experience debilitating muscle weakness, resulting in impaired mobility, speech, swallowing, vision and respiratory function. Efgartigimod is a human IgG1 antibody Fc fragment engineered for increased binding affinity to neonatal Fc receptor. The neonatal Fc receptor blockade by efgartigimod competitively inhibits endogenous IgG binding, leading to decreased IgG recycling and increased degradation resulting in lower IgG concentration.
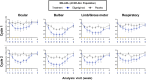
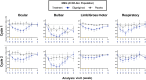
Methods: The safety and efficacy of efgartigimod were evaluated in the ADAPT study. Key efficacy outcome measures included Myasthenia Gravis Activities of Daily Living (MG-ADL) and Quantitative Myasthenia Gravis (QMG) scores. Efgartigimod demonstrated significant improvement in both the MG-ADL and QMG scores. This post hoc analysis aimed to determine whether all subdomains of MG-ADL and QMG improved with efgartigimod treatment. Individual items of MG-ADL and QMG were grouped into four subdomains: bulbar, ocular, limb/gross motor and respiratory. Change from baseline over 10 weeks in each subdomain was calculated for each group.
Results: Greater improvements from baseline were seen across MG-ADL subdomains in participants treated with efgartigimod compared with placebo. These improvements were typically observed 1 to 2 weeks after the first infusion and correlated with reductions in IgG. Similar results were observed across most QMG subdomains.
Conclusions: These post hoc analyses of MG-ADL and QMG subdomain data from ADAPT suggest that efgartigimod is beneficial in improving muscle function and strength across all muscle groups, leading to the observed efficacy in participants with gMG.
Keywords: ADAPT; efgartigimod; generalized myasthenia gravis; immunoglobulin G; neonatal Fc receptor.
© 2023 The Authors. European Journal of Neurology published by John Wiley & Sons Ltd on behalf of European Academy of Neurology.
Conflict of interest statement
VB has received research support from AZ‐Alexion, Grifols, CSL, UCB, argenx, Takeda, Octapharma, Akcea, Momenta (J&J), Immunovant, Ionis and Viela. JFH has received research support (paid to his institution) from Alexion Pharmaceuticals Inc., argenx, Cartesian Therapeutics, the Centers for Disease Control and Prevention, Myasthenia Gravis Foundation of America, Muscular Dystrophy Association, National Institutes of Health (including the National Institute of Neurological Disorders and Stroke and the National Institute of Arthritis and Musculoskeletal and Skin Diseases), Patient‐Centered Outcomes Research Institute, Ra Pharmaceuticals Inc. (now UCB), honoraria from AcademicCME, Alexion Pharmaceuticals Inc., argenx, Biologix Pharma, F. Hoffman LaRoche Ltd, Horizon Therapeutics plc, Medscape CME, Merck EMB Serono, NMD Pharma, Novartis Pharmaceuticals, PeerView CME, Ra Pharmaceuticals Inc. (now UCB), Regeneron Pharmaceuticals Inc., Sanofi US and Zai Labs; and nonfinancial support from Alexion Pharmaceuticals Inc., argenx, Ra Pharmaceuticals Inc. (now UCB) and Toleranzia AB. CK served as a deputy editor for
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

