Pathophysiological changes of muscle after ischemic stroke: a secondary consequence of stroke injury
- PMID: 37843207
- PMCID: PMC10664100
- DOI: 10.4103/1673-5374.382221
Pathophysiological changes of muscle after ischemic stroke: a secondary consequence of stroke injury
Abstract
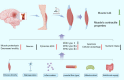
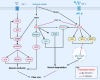
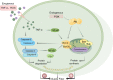
Sufficient clinical evidence suggests that the damage caused by ischemic stroke to the body occurs not only in the acute phase but also during the recovery period, and that the latter has a greater impact on the long-term prognosis of the patient. However, current stroke studies have typically focused only on lesions in the central nervous system, ignoring secondary damage caused by this disease. Such a phenomenon arises from the slow progress of pathophysiological studies examining the central nervous system. Further, the appropriate therapeutic time window and benefits of thrombolytic therapy are still controversial, leading scholars to explore more pragmatic intervention strategies. As treatment measures targeting limb symptoms can greatly improve a patient's quality of life, they have become a critical intervention strategy. As the most vital component of the limbs, skeletal muscles have become potential points of concern. Despite this, to the best of our knowledge, there are no comprehensive reviews of pathophysiological changes and potential treatments for post-stroke skeletal muscle. The current review seeks to fill a gap in the current understanding of the pathological processes and mechanisms of muscle wasting atrophy, inflammation, neuroregeneration, mitochondrial changes, and nutritional dysregulation in stroke survivors. In addition, the challenges, as well as the optional solutions for individualized rehabilitation programs for stroke patients based on motor function are discussed.
Keywords: inflammation; ischemic stroke; mitochondria; muscle atrophy; muscle fiber; muscle nutrition; quality of life; rehabilitation; ubiquitin.
Conflict of interest statement
None
Figures
References
-
- Amantea D, Nappi G, Bernardi G, Bagetta G, Corasaniti MT. Post-ischemic brain damage: pathophysiology and role of inflammatory mediators. FEBS J. 2009;276:13–26. - PubMed
-
- Aoyama S, Kim HK, Hirooka R, Tanaka M, Shimoda T, Chijiki H, Kojima S, Sasaki K, Takahashi K, Makino S, Takizawa M, Takahashi M, Tahara Y, Shimba S, Shinohara K, Shibata S. Distribution of dietary protein intake in daily meals influences skeletal muscle hypertrophy via the muscle clock. Cell Rep. 2021;36:109336. - PubMed
-
- Arasaki K, Igarashi O, Ichikawa Y, Machida T, Shirozu I, Hyodo A, Ushijima R. Reduction in the motor unit number estimate (MUNE) after cerebral infarction. J Neurol Sci. 2006;250:27–32. - PubMed
-
- Babyar SR, White H, Shafi N, Reding M. Outcomes with stroke and lateropulsion: a case-matched controlled study. Neurorehabil Neural Repair. 2008;22:415–423. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

