Activation of cerebral Ras-related C3 botulinum toxin substrate (Rac) 1 promotes post-ischemic stroke functional recovery in aged mice
- PMID: 37843224
- PMCID: PMC10664129
- DOI: 10.4103/1673-5374.382256
Activation of cerebral Ras-related C3 botulinum toxin substrate (Rac) 1 promotes post-ischemic stroke functional recovery in aged mice
Abstract
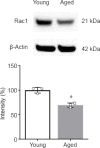
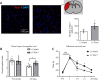
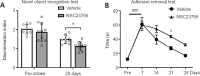
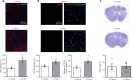
Brain functional impairment after stroke is common; however, the molecular mechanisms of post-stroke recovery remain unclear. It is well-recognized that age is the most important independent predictor of poor outcomes after stroke as older patients show poorer functional outcomes following stroke. Mounting evidence suggests that axonal regeneration and angiogenesis, the major forms of brain plasticity responsible for post-stroke recovery, diminished with advanced age. Previous studies suggest that Ras-related C3 botulinum toxin substrate (Rac) 1 enhances stroke recovery as activation of Rac1 improved behavior recovery in a young mice stroke model. Here, we investigated the role of Rac1 signaling in long-term functional recovery and brain plasticity in an aged (male, 18 to 22 months old C57BL/6J) brain after ischemic stroke. We found that as mice aged, Rac1 expression declined in the brain. Delayed overexpression of Rac1, using lentivirus encoding Rac1 injected day 1 after ischemic stroke, promoted cognitive (assessed using novel object recognition test) and sensorimotor (assessed using adhesive removal tests) recovery on days 14-28. This was accompanied by the increase of neurite and proliferative endothelial cells in the peri-infarct zone assessed by immunostaining. In a reverse approach, pharmacological inhibition of Rac1 by intraperitoneal injection of Rac1 inhibitor NSC23766 for 14 successive days after ischemic stroke worsened the outcome with the reduction of neurite and proliferative endothelial cells. Furthermore, Rac1 inhibition reduced the activation of p21-activated kinase 1, the protein level of brain-derived neurotrophic factor, and increased the protein level of glial fibrillary acidic protein in the ischemic brain on day 28 after stroke. Our work provided insight into the mechanisms behind the diminished plasticity after cerebral ischemia in aged brains and identified Rac1 as a potential therapeutic target for improving functional recovery in the older adults after stroke.
Keywords: Pak1; Rac1; aging; angiogenesis; brain-derived neurotrophic factor (BDNF); cerebral ischemia; cognitive recovery; neurite; sensorimotor recovery; stroke.
Conflict of interest statement
None
Figures


Similar articles
-
Activation of neuronal Ras-related C3 botulinum toxin substrate 1 (Rac1) improves post-stroke recovery and axonal plasticity in mice.J Neurochem. 2021 May;157(4):1366-1376. doi: 10.1111/jnc.15195. Epub 2020 Oct 6. J Neurochem. 2021. PMID: 32964455 Free PMC article.
-
Activation of endothelial ras-related C3 botulinum toxin substrate 1 (Rac1) improves post-stroke recovery and angiogenesis via activating Pak1 in mice.Exp Neurol. 2019 Dec;322:113059. doi: 10.1016/j.expneurol.2019.113059. Epub 2019 Sep 6. Exp Neurol. 2019. PMID: 31499064 Free PMC article. Review.
-
Ras-Related C3 Botulinum Toxin Substrate 1 Promotes Axonal Regeneration after Stroke in Mice.Transl Stroke Res. 2018 Oct;9(5):506-514. doi: 10.1007/s12975-018-0611-5. Epub 2018 Feb 24. Transl Stroke Res. 2018. PMID: 29476448 Free PMC article.
-
Citalopram enhances neurovascular regeneration and sensorimotor functional recovery after ischemic stroke in mice.Neuroscience. 2013 Sep 5;247:1-11. doi: 10.1016/j.neuroscience.2013.04.011. Epub 2013 Apr 13. Neuroscience. 2013. PMID: 23590907 Free PMC article.
-
Rac-1 as a new therapeutic target in cerebro- and cardio-vascular diseases.Curr Drug Targets. 2014;15(13):1231-46. doi: 10.2174/1389450115666141027110156. Curr Drug Targets. 2014. PMID: 25345393 Review.
Cited by
-
Targeted TGF-βR2 Knockdown in the Retrotrapezoid Nucleus Mitigates Respiratory Dysfunction and Cognitive Decline in a Mouse Model of Cerebral Amyloid Angiopathy with and without Stroke.Res Sq [Preprint]. 2024 May 31:rs.3.rs-4438544. doi: 10.21203/rs.3.rs-4438544/v1. Res Sq. 2024. Update in: Transl Stroke Res. 2025 Aug;16(4):1272-1284. doi: 10.1007/s12975-024-01306-0. PMID: 38854014 Free PMC article. Updated. Preprint.
-
Topological functional network analysis of cortical blood flow in hyperacute ischemic rats.Brain Struct Funct. 2024 Dec 26;230(1):20. doi: 10.1007/s00429-024-02864-7. Brain Struct Funct. 2024. PMID: 39724244 Free PMC article.
-
Insights into spinal muscular atrophy from molecular biomarkers.Neural Regen Res. 2025 Jul 1;20(7):1849-1863. doi: 10.4103/NRR.NRR-D-24-00067. Epub 2024 Jun 26. Neural Regen Res. 2025. PMID: 38934395 Free PMC article.
-
Targeted TGF-βR2 Silencing in the Retrotrapezoid Nucleus Mitigates Respiratory Dysfunction and Cognitive Decline in a Mouse Model of Cerebral Amyloid Angiopathy with and without Stroke.Transl Stroke Res. 2025 Aug;16(4):1272-1284. doi: 10.1007/s12975-024-01306-0. Epub 2024 Nov 14. Transl Stroke Res. 2025. PMID: 39543011
-
Integrated bioinformatics analysis and biological experiments to identify key immune genes in vascular dementia.Front Immunol. 2025 Mar 24;16:1560438. doi: 10.3389/fimmu.2025.1560438. eCollection 2025. Front Immunol. 2025. PMID: 40196107 Free PMC article.
References
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

