SPTAN1/NUMB axis senses cell density to restrain cell growth and oncogenesis through Hippo signaling
- PMID: 37843276
- PMCID: PMC10575737
- DOI: 10.1172/JCI168888
SPTAN1/NUMB axis senses cell density to restrain cell growth and oncogenesis through Hippo signaling
Abstract
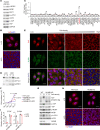
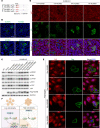
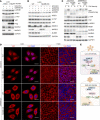
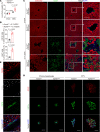
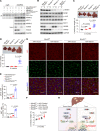
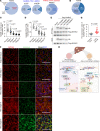
The loss of contact inhibition is a key step during carcinogenesis. The Hippo-Yes-associated protein (Hippo/YAP) pathway is an important regulator of cell growth in a cell density-dependent manner. However, how Hippo signaling senses cell density in this context remains elusive. Here, we report that high cell density induced the phosphorylation of spectrin α chain, nonerythrocytic 1 (SPTAN1), a plasma membrane-stabilizing protein, to recruit NUMB endocytic adaptor protein isoforms 1 and 2 (NUMB1/2), which further sequestered microtubule affinity-regulating kinases (MARKs) in the plasma membrane and rendered them inaccessible for phosphorylation and inhibition of the Hippo kinases sterile 20-like kinases MST1 and MST2 (MST1/2). WW45 interaction with MST1/2 was thereby enhanced, resulting in the activation of Hippo signaling to block YAP activity for cell contact inhibition. Importantly, low cell density led to SPTAN1 dephosphorylation and NUMB cytoplasmic location, along with MST1/2 inhibition and, consequently, YAP activation. Moreover, double KO of NUMB and WW45 in the liver led to appreciable organ enlargement and rapid tumorigenesis. Interestingly, NUMB isoforms 3 and 4, which have a truncated phosphotyrosine-binding (PTB) domain and are thus unable to interact with phosphorylated SPTAN1 and activate MST1/2, were selectively upregulated in liver cancer, which correlated with YAP activation. We have thus revealed a SPTAN1/NUMB1/2 axis that acts as a cell density sensor to restrain cell growth and oncogenesis by coupling external cell-cell contact signals to intracellular Hippo signaling.
Keywords: Cancer; Cell Biology; Liver cancer; Oncology; Signal transduction.
Figures




References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

