Angiopoietin-2 blockade suppresses growth of liver metastases from pancreatic neuroendocrine tumors by promoting T cell recruitment
- PMID: 37843277
- PMCID: PMC10575726
- DOI: 10.1172/JCI167994
Angiopoietin-2 blockade suppresses growth of liver metastases from pancreatic neuroendocrine tumors by promoting T cell recruitment
Abstract
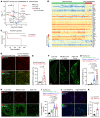
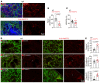
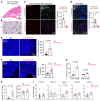
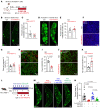
Improving the management of metastasis in pancreatic neuroendocrine tumors (PanNETs) is critical, as nearly half of patients with PanNETs present with liver metastases, and this accounts for the majority of patient mortality. We identified angiopoietin-2 (ANGPT2) as one of the most upregulated angiogenic factors in RNA-Seq data from human PanNET liver metastases and found that higher ANGPT2 expression correlated with poor survival rates. Immunohistochemical staining revealed that ANGPT2 was localized to the endothelial cells of blood vessels in PanNET liver metastases. We observed an association between the upregulation of endothelial ANGPT2 and liver metastatic progression in both patients and transgenic mouse models of PanNETs. In human and mouse PanNET liver metastases, ANGPT2 upregulation coincided with poor T cell infiltration, indicative of an immunosuppressive tumor microenvironment. Notably, both pharmacologic inhibition and genetic deletion of ANGPT2 in PanNET mouse models slowed the growth of PanNET liver metastases. Furthermore, pharmacologic inhibition of ANGPT2 promoted T cell infiltration and activation in liver metastases, improving the survival of mice with metastatic PanNETs. These changes were accompanied by reduced plasma leakage and improved vascular integrity in metastases. Together, these findings suggest that ANGPT2 blockade may be an effective strategy for promoting T cell infiltration and immunostimulatory reprogramming to reduce the growth of liver metastases in PanNETs.
Keywords: Angiogenesis; T cells.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

