Parental measurement of height in growth hormone-treated children in the hospital setting proves valid: an observational study - potential for replacement of outpatient clinic visits to the home setting
- PMID: 37843612
- PMCID: PMC10951002
- DOI: 10.1007/s00431-023-05232-5
Parental measurement of height in growth hormone-treated children in the hospital setting proves valid: an observational study - potential for replacement of outpatient clinic visits to the home setting
Abstract
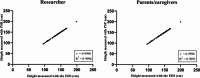
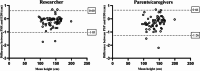
Reliable height measurement plays a pivotal role in evaluating the efficacy of costly growth hormone (GH) therapy in children. Currently, regularly outpatient clinic visits are needed to accurately measure height. The outpatient clinic visits are time-consuming for parents as well for health care professionals. This observational study aimed to investigate the validity of parentally performed height measurements compared to height measurements in the outpatient setting. An observational study was performed at the outpatient clinic of Amalia's Children's Hospital Nijmegen. A portable stadiometer (PS) was developed for height measurements at home. Measurements with the PS were performed by the researcher (PSR) and parents/caregivers (PSP). Measurements performed with the electronic digital ruler (EDS) were considered as the gold standard. The parents were potentially unblinded for the gold standard measurement (EDS). Descriptive statistics, Wilcoxon signed-rank, and Pearson's correlation tests were performed. The Bland-Altman plots were made to illustrate the correlation of the PSR or PSP with the gold standard. The correlation between the height measurements with PSR or PSP compared to the EDS was substantial (PSR: r = 0.9998, R2 = 0.9996, P < 0.001; PSP: r = 0.9998, R2 = 0.9995, P < 0.001). However, a statistically significant underestimation of the PSR and PSP was observed (P < 0.001). The mean difference of the PSR and PSP was respectively - 0.21 cm ± 0.52 SD and - 0.30 cm ± 0.62 SD in comparison to the EDS. The Bland-Altman plots illustrated that 95% of the PSR measurements were between - 1.03 and 0.60 cm and 95% of the PSP measurements were between - 1.26 and 0.66 cm compared to the EDS.
Conclusion: We found a strong correlation between the PSR or PSP and the EDS, with only a minor underestimation of approximately 0.2-0.3 cm. In our opinion, this underestimation is clinically irrelevant as it does not result in an adjustment in GH dose. To conclude, parental height measurements could be a promising tool as it partially replaces outpatient clinic visits needed for measurements of height. Further studies are required to confirm this statement.
What is known: • The immense impact of the coronavirus disease 2019 (COVID-19) pandemic on health care has increased telemedicine worldwide. For adequate integration of telemedicine in paediatric growth hormone treatment, reliable height and weight measurements in the home setting are required. • Earlier studies have shown that parents are capable to reliable perform height measurements in healthy children.
What is new: • To our knowledge, this is the first study to show a strong correlation between the height measurements with a portable stadiometer by parents and those made with the electronic digital ruler (gold standard) in children treated with growth hormone. There was only a minor underestimation of approximately 0.2-0.3 cm, which we anticipated to be clinically irrelevant. • Therefore, home height measurements can at least partly replace costly outpatient visits for children being treated with growth hormone as part of an uncomplicated course. Moreover, these results may also be promising for implementation in other paediatric populations besides children treated with growth hormone.
Keywords: Height; Parentally reported; Portable stadiometer; Transitioning outpatient visits to home care; Validity.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures

References
-
- Orso M, Polistena B, Granato S, Novelli G, Di Virgilio R, La Torre D, d’Angela D, Spandonaro F. Pediatric growth hormone treatment in Italy: a systematic review of epidemiology, quality of life, treatment adherence, and economic impact. PLoS ONE. 2022;17:e0264403. doi: 10.1371/journal.pone.0264403. - DOI - PMC - PubMed
-
- National Institute for Health and Care Excellence (2010) Human growth hormone (somatropin) for the treatment of growth failure in children. NICE technology appraisal guidance [TA188]
-
- Dutch National Registry of Growth Hormone treatment in children (2018) Annual report of 2018. Rotterdam
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

