A Rab6 to Rab11 transition is required for dense-core granule and exosome biogenesis in Drosophila secondary cells
- PMID: 37844085
- PMCID: PMC10602379
- DOI: 10.1371/journal.pgen.1010979
A Rab6 to Rab11 transition is required for dense-core granule and exosome biogenesis in Drosophila secondary cells
Abstract
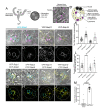
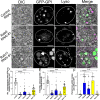
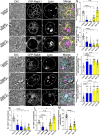
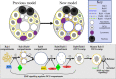
Secretory cells in glands and the nervous system frequently package and store proteins destined for regulated secretion in dense-core granules (DCGs), which disperse when released from the cell surface. Despite the relevance of this dynamic process to diseases such as diabetes and human neurodegenerative disorders, our mechanistic understanding is relatively limited, because of the lack of good cell models to follow the nanoscale events involved. Here, we employ the prostate-like secondary cells (SCs) of the Drosophila male accessory gland to dissect the cell biology and genetics of DCG biogenesis. These cells contain unusually enlarged DCGs, which are assembled in compartments that also form secreted nanovesicles called exosomes. We demonstrate that known conserved regulators of DCG biogenesis, including the small G-protein Arf1 and the coatomer complex AP-1, play key roles in making SC DCGs. Using real-time imaging, we find that the aggregation events driving DCG biogenesis are accompanied by a change in the membrane-associated small Rab GTPases which are major regulators of membrane and protein trafficking in the secretory and endosomal systems. Indeed, a transition from trans-Golgi Rab6 to recycling endosomal protein Rab11, which requires conserved DCG regulators like AP-1, is essential for DCG and exosome biogenesis. Our data allow us to develop a model for DCG biogenesis that brings together several previously disparate observations concerning this process and highlights the importance of communication between the secretory and endosomal systems in controlling regulated secretion.
Copyright: © 2023 Wells et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures





Similar articles
-
Regulation of Dense-Core Granule Replenishment by Autocrine BMP Signalling in Drosophila Secondary Cells.PLoS Genet. 2016 Oct 11;12(10):e1006366. doi: 10.1371/journal.pgen.1006366. eCollection 2016 Oct. PLoS Genet. 2016. PMID: 27727275 Free PMC article.
-
A novel function for Rab1 and Rab11 during secretory granule maturation.J Cell Sci. 2021 Aug 1;134(15):jcs259037. doi: 10.1242/jcs.259037. Epub 2021 Aug 3. J Cell Sci. 2021. PMID: 34342349 Free PMC article.
-
Protease nexin-1 promotes secretory granule biogenesis by preventing granule protein degradation.Mol Biol Cell. 2006 Feb;17(2):789-98. doi: 10.1091/mbc.e05-08-0755. Epub 2005 Nov 30. Mol Biol Cell. 2006. PMID: 16319172 Free PMC article.
-
Chromogranin A: a new proposal for trafficking, processing and induction of granule biogenesis.Regul Pept. 2010 Feb 25;160(1-3):153-9. doi: 10.1016/j.regpep.2009.12.007. Epub 2009 Dec 16. Regul Pept. 2010. PMID: 20006653 Free PMC article. Review.
-
Endosomal recycling protein Rab11 in Parkin and Pink1 signaling in Drosophila model of Parkinson's disease.Exp Cell Res. 2022 Nov 15;420(2):113357. doi: 10.1016/j.yexcr.2022.113357. Epub 2022 Sep 15. Exp Cell Res. 2022. PMID: 36116557 Review.
Cited by
-
Trafficking of hormones and trophic factors to secretory and extracellular vesicles: a historical perspective and new hypothesis.Extracell Vesicles Circ Nucl Acids. 2023;4(4):568-587. doi: 10.20517/evcna.2023.34. Epub 2023 Nov 9. Extracell Vesicles Circ Nucl Acids. 2023. PMID: 38435713 Free PMC article.
-
Amyloid-β disrupts APP-regulated protein aggregation and dissociation from recycling endosomal membranes.EMBO J. 2025 Aug;44(16):4443-4472. doi: 10.1038/s44318-025-00497-y. Epub 2025 Jul 17. EMBO J. 2025. PMID: 40676215 Free PMC article.
-
ARF1 compartments direct cargo flow via maturation into recycling endosomes.Nat Cell Biol. 2024 Nov;26(11):1845-1859. doi: 10.1038/s41556-024-01518-4. Epub 2024 Oct 4. Nat Cell Biol. 2024. PMID: 39367144 Free PMC article.
-
RAB-10 cooperates with EHBP-1 to capture vesicular carriers during post-Golgi exocytic trafficking.J Cell Biol. 2025 Apr 7;224(4):e202410003. doi: 10.1083/jcb.202410003. Epub 2025 Feb 21. J Cell Biol. 2025. PMID: 39982707
-
Two roads diverged in a cell: insights from differential exosome regulation in polarized cells.Front Cell Dev Biol. 2024 Sep 2;12:1451988. doi: 10.3389/fcell.2024.1451988. eCollection 2024. Front Cell Dev Biol. 2024. PMID: 39286483 Free PMC article. Review.
References
-
- Tsuchiya M, Hosaka M, Moriguchi T, Zhang S, Suda M, Yokota-Hashimoto H, et al.. Cholesterol biosynthesis pathway intermediates and inhibitors regulate glucose-stimulated insulin secretion and secretory granule formation in pancreatic beta-cells. Endocrinology. 2010;151: 4705–16. doi: 10.1210/en.2010-0623 - DOI - PubMed
-
- Giudici AM, Sher E, Pelagi M, Clementi F, Zanini A. Immunolocalization of secretogranin II, chromogranin A, and chromogranin B in differentiating human neuroblastoma cells. Eur J Cell Biol. 1992;58: 383–9. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

